The Lancet Oncology ( IF 41.6 ) Pub Date : 2020-03-11 , DOI: 10.1016/s1470-2045(20)30059-0 Kazuyuki Shimada 1 , Motoko Yamaguchi 2 , Yoshiko Atsuta 3 , Kosei Matsue 4 , Keijiro Sato 5 , Shigeru Kusumoto 6 , Hirokazu Nagai 7 , Jun Takizawa 8 , Noriko Fukuhara 9 , Koji Nagafuji 10 , Kana Miyazaki 2 , Eiichi Ohtsuka 11 , Masataka Okamoto 12 , Yasumasa Sugita 13 , Toshiki Uchida 14 , Satoshi Kayukawa 15 , Atsushi Wake 16 , Daisuke Ennishi 17 , Yukio Kondo 18 , Tohru Izumi 19 , Yoshihiro Kin 20 , Kunihiro Tsukasaki 21 , Daigo Hashimoto 22 , Masaaki Yuge 23 , Atsumi Yanagisawa 3 , Yachiyo Kuwatsuka 24 , Satoko Shimada 25 , Yasufumi Masaki 26 , Nozomi Niitsu 27 , Hitoshi Kiyoi 1 , Ritsuro Suzuki 28 , Takashi Tokunaga 7 , Shigeo Nakamura 25 , Tomohiro Kinoshita 29
|
Background
Intravascular large B-cell lymphoma (IVLBCL) is a rare disease for which there is no available standard treatment. We aimed to ascertain the safety and activity of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) with high-dose methotrexate and intrathecal chemotherapy as CNS-oriented therapy for patients with previously untreated IVLBCL.
Methods
PRIMEUR-IVL is a multicentre, single-arm, phase 2 trial at 22 hospitals in Japan. Eligible patients had untreated histologically confirmed IVLBCL, were aged 20–79 years, had an Eastern Cooperative Group performance status of 0–3, and had no apparent CNS involvement at diagnosis. Patients received three cycles of R-CHOP (rituximab 375 mg/m2 intravenously on day 1 [except cycle one, which was on day 8]; cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1·4 mg/m2 [maximum 2·0 mg] intravenously on day 1 of cycle one and day 2 of cycles two and three; and prednisolone 100 mg/day orally on days 1–5 of cycle one and days 2–6 of cycles two and three) followed by two cycles of rituximab with high-dose methotrexate (3·5 g/m2 intravenously on day 2 of cycles four and five) every 2 weeks and three additional cycles of R-CHOP. Intrathecal chemotherapy (methotrexate 15 mg, cytarabine 40 mg, and prednisolone 10 mg) was administered four times during the R-CHOP phase. The primary endpoint was 2-year progression-free survival. Efficacy analyses were done in all enrolled patients; safety analyses were done in all enrolled and treated patients. The trial is registered in the UMIN Clinical Trials Registry (UMIN000005707) and the Japan Registry of Clinical Trials (jRCTs041180165); the trial is ongoing for long-term follow-up.
Findings
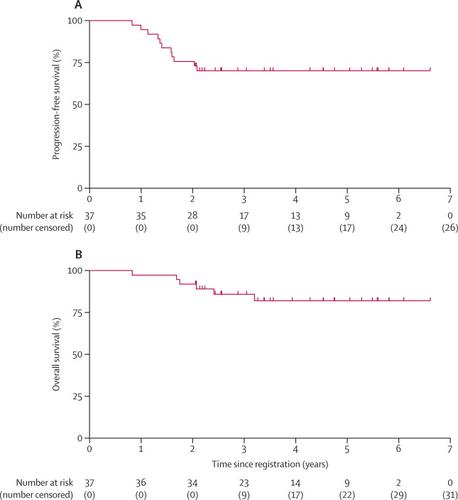
Between June 16, 2011, and July 21, 2016, 38 patients were enrolled, of whom 37 were eligible; one patient was excluded because of a history of testicular lymphoma. Median follow-up was 3·9 years (IQR 2·5–5·5). 2-year progression-free survival was 76% (95% CI 58–87). The most frequent adverse events of grade 3–4 were neutropenia and leucocytopenia, which were reported in all 38 (100%) patients. Serious adverse events were hypokalaemia, febrile neutropenia with hypotension, hypertension, and intracerebral haemorrhage (reported in one [3%] patient each). No treatment-related deaths occurred during protocol treatment.
Interpretation
R-CHOP combined with rituximab and high-dose methotrexate plus intrathecal chemotherapy is a safe and active treatment for patients with IVLBCL without apparent CNS involvement at diagnosis, and this regimen warrants future investigation.
Funding
The Japan Agency for Medical Research and Development, the Center for Supporting Hematology-Oncology Trials, and the National Cancer Center.
中文翻译:

利妥昔单抗,环磷酰胺,阿霉素,长春新碱和泼尼松龙联合大剂量甲氨蝶呤加鞘内化疗联合治疗新诊断的血管内大B细胞淋巴瘤(PRIMEUR-IVL):一项多中心,单臂,2期试验。
背景
血管内大B细胞淋巴瘤(IVLBCL)是一种罕见疾病,目前尚无标准治疗方法。我们旨在确定高剂量甲氨蝶呤和鞘内化疗作为中枢神经系统导向疗法对以前未经治疗的IVLBCL患者的R-CHOP(利妥昔单抗,环磷酰胺,阿霉素,长春新碱和泼尼松龙)的安全性和活性。
方法
PRIMEUR-IVL是一项在日本的22家医院进行的多中心单臂2期临床试验。符合条件的患者未经组织学证实的IVLBCL治疗,年龄20-79岁,东部合作组织的表现为0-3,诊断中无明显的CNS参与。患者在第1天接受了三个周期的R-CHOP(利妥昔单抗375 mg / m 2静脉注射[第一周期除外,在第8天除外);环磷酰胺750 mg / m 2,阿霉素50 mg / m 2和长春新碱1·4在第一个周期的第1天以及第2和第三个周期的第2天静脉内注射mg / m 2 [最大2·0 mg];在第一个周期的第1-5天和第二个周期的第2-6天口服泼尼松龙100 mg /天和三个),然后是两个周期的利妥昔单抗与大剂量甲氨蝶呤(3·5 g / m2静脉内上个周期四个和五个)每2周和R-CHOP的附加三个周期的第2天。在R-CHOP阶段进行四次鞘内化疗(甲氨蝶呤15 mg,阿糖胞苷40 mg,泼尼松龙10 mg)。主要终点是2年无进展生存期。对所有入组患者进行功效分析;对所有入组和治疗的患者进行安全性分析。该试验已在UMIN临床试验注册中心(UMIN000005707)和日本临床试验注册中心(jRCTs041180165)中注册;该试验正在进行长期随访。
发现
在2011年6月16日至2016年7月21日期间,共有38例患者入选,其中37例符合条件。一名患者因睾丸淋巴瘤病史被排除在外。中位随访时间为3·9年(IQR 2·5-5·5)。2年无进展生存率为76%(95%CI 58-87)。3-4级患者最常见的不良事件是中性粒细胞减少和白细胞减少,在所有38例(100%)患者中均有报道。严重的不良事件为低钾血症,伴有低血压的发热性中性粒细胞减少,高血压和脑内出血(每例[3%]患者报告)。在方案治疗期间,未发生与治疗相关的死亡。
解释
R-CHOP联合利妥昔单抗和大剂量甲氨蝶呤加鞘内化疗是对IVLBCL患者的一种安全有效的治疗方法,在诊断中没有明显的CNS参与,该方案值得进一步研究。
资金
日本医学研究与发展局,血液肿瘤试验支持中心和国家癌症中心。










































 京公网安备 11010802027423号
京公网安备 11010802027423号