当前位置:
X-MOL 学术
›
JACC Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Natriuretic Peptide-Based Inclusion Criteria in a Heart Failure Clinical Trial: Insights From COMMANDER HF.
JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-03-11 , DOI: 10.1016/j.jchf.2019.12.009 Jonathan W Cunningham 1 , João Pedro Ferreira 2 , Hsiaowei Deng 3 , Stefan D Anker 4 , William M Byra 3 , John G F Cleland 5 , Mihai Gheorghiade 6 , Carolyn S P Lam 7 , David La Police 3 , Mandeep R Mehra 1 , James D Neaton 8 , Theodore E Spiro 9 , Dirk J van Veldhuisen 10 , Barry Greenberg 11 , Faiez Zannad 2
JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-03-11 , DOI: 10.1016/j.jchf.2019.12.009 Jonathan W Cunningham 1 , João Pedro Ferreira 2 , Hsiaowei Deng 3 , Stefan D Anker 4 , William M Byra 3 , John G F Cleland 5 , Mihai Gheorghiade 6 , Carolyn S P Lam 7 , David La Police 3 , Mandeep R Mehra 1 , James D Neaton 8 , Theodore E Spiro 9 , Dirk J van Veldhuisen 10 , Barry Greenberg 11 , Faiez Zannad 2
Affiliation
|
OBJECTIVES
This study investigated the effects of a mid-trial protocol amendment requiring elevated natriuretic peptides for inclusion in the COMMANDER-HF (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure) trial.
BACKGROUND
Heart failure (HF) trials that select patients based on history of HF hospitalization alone are susceptible to regional variations in event rates. Elevated plasma concentrations of natriuretic peptides (NPs) as selection criteria may help HF ascertainment and risk enrichment. In the COMMANDER-HF trial, B-type natriuretic peptide ≥200 ng/l or N-terminal pro-B-type natriuretic peptide ≥800 ng/l were added to inclusion criteria as a mid-trial protocol amendment, providing a unique case-study of NP-based inclusion criteria.
METHODS
We compared the baseline characteristics, event rates, and treatment effects for patients enrolled before and after the NP protocol amendment. The primary endpoint was all-cause death, myocardial infarction, or stroke. Secondary endpoints included HF rehospitalization and cardiovascular death.
RESULTS
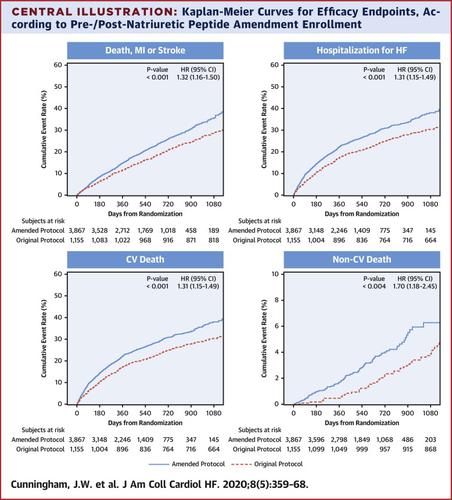
A total of 5,022 patients with left ventricular ejection fraction ≤40% and coronary artery disease were included. Compared to patients enrolled before the NP protocol amendment, those enrolled post-amendment (n = 3,867, 77%) were older, more often had diabetes, and had lower values for body mass index, left ventricular ejection fraction, and estimated glomerular filtration rate, higher heart rate, and higher event rates: primary endpoint (hazard ratio [HR]: 1.32; 95% confidence interval [CI]: 1.16 to 1.50), cardiovascular death (HR: 1.29; 95% CI: 1.11 to 1.50), HF rehospitalization (HR: 1.31; 95% CI: 1.15 to 1.49), and major bleeding (HR: 1.71; 95% CI: 1.11 to 2.65). Differences between pre- and post-amendment rates were confined to and driven by Eastern Europe. This protocol amendment did not modify the neutral effect of rivaroxaban on the primary endpoint (p interaction = 0.36) or secondary endpoints.
CONCLUSIONS
In a global event-driven trial of rivaroxaban in HF, requiring elevated NPs for inclusion increased event rates allowing earlier completion of the trial but did not modify treatment effect. These data inform future HF trials regarding the expected impact of NP-based inclusion criteria on patient characteristics and event rates. (COMMANDER HF [A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure] NCT01877915).
中文翻译:

心力衰竭临床试验中基于利尿钠肽的纳入标准:COMMANDER HF 的见解。
目的 本研究调查了试验中期方案修正案的影响,该修正案要求将利钠肽纳入 COMMANDER-HF(一项评估利伐沙班在降低患有以下疾病的参与者中死亡、心肌梗死或中风风险方面的有效性和安全性的研究):失代偿性心力衰竭发作后的心力衰竭和冠状动脉疾病)试验。背景仅根据心力衰竭住院史选择患者的心力衰竭(HF)试验容易受到事件发生率的区域差异的影响。将利钠肽 (NP) 血浆浓度升高作为选择标准可能有助于心力衰竭的确定和风险丰富。在 COMMANDER-HF 试验中,B 型利钠肽≥200 ng/l 或 N 端 B 型利钠肽原≥800 ng/l 被添加到纳入标准中,作为试验中期方案修订,提供了一个独特的案例-基于NP的纳入标准的研究。方法 我们比较了 NP 方案修订前后入组患者的基线特征、事件发生率和治疗效果。主要终点是全因死亡、心肌梗死或中风。次要终点包括心力衰竭再住院和心血管死亡。结果共纳入5022例左心室射血分数≤40%且患有冠心病的患者。与 NP 方案修订前入组的患者相比,修订后入组的患者 (n = 3,867, 77%) 年龄更大,更常患有糖尿病,并且体重指数、左心室射血分数和估计肾小球滤过率的值较低、更高的心率和更高的事件发生率:主要终点(风险比 [HR]:1.32;95% 置信区间 [CI]:1.16 至 1.50)、心血管死亡(HR:1.29; 95% CI:1.11 至 1.50)、心衰再住院(HR:1.31;95% CI:1.15 至 1.49)和大出血(HR:1.71;95% CI:1.11 至 2.65)。修订前和修订后利率之间的差异仅限于东欧并由东欧驱动。该方案修订并未改变利伐沙班对主要终点(p 相互作用 = 0.36)或次要终点的中性作用。结论 在利伐沙班治疗心力衰竭的全球事件驱动试验中,要求纳入更高的 NP 会增加事件发生率,从而允许试验提前完成,但不会改变治疗效果。这些数据为未来的心力衰竭试验提供了有关基于 NP 的纳入标准对患者特征和事件发生率的预期影响的信息。 (COMMANDER HF [一项评估利伐沙班降低失代偿性心力衰竭发作后患有心力衰竭和冠状动脉疾病的参与者死亡、心肌梗塞或中风风险的有效性和安全性的研究] NCT01877915)。
更新日期:2020-03-11
中文翻译:

心力衰竭临床试验中基于利尿钠肽的纳入标准:COMMANDER HF 的见解。
目的 本研究调查了试验中期方案修正案的影响,该修正案要求将利钠肽纳入 COMMANDER-HF(一项评估利伐沙班在降低患有以下疾病的参与者中死亡、心肌梗死或中风风险方面的有效性和安全性的研究):失代偿性心力衰竭发作后的心力衰竭和冠状动脉疾病)试验。背景仅根据心力衰竭住院史选择患者的心力衰竭(HF)试验容易受到事件发生率的区域差异的影响。将利钠肽 (NP) 血浆浓度升高作为选择标准可能有助于心力衰竭的确定和风险丰富。在 COMMANDER-HF 试验中,B 型利钠肽≥200 ng/l 或 N 端 B 型利钠肽原≥800 ng/l 被添加到纳入标准中,作为试验中期方案修订,提供了一个独特的案例-基于NP的纳入标准的研究。方法 我们比较了 NP 方案修订前后入组患者的基线特征、事件发生率和治疗效果。主要终点是全因死亡、心肌梗死或中风。次要终点包括心力衰竭再住院和心血管死亡。结果共纳入5022例左心室射血分数≤40%且患有冠心病的患者。与 NP 方案修订前入组的患者相比,修订后入组的患者 (n = 3,867, 77%) 年龄更大,更常患有糖尿病,并且体重指数、左心室射血分数和估计肾小球滤过率的值较低、更高的心率和更高的事件发生率:主要终点(风险比 [HR]:1.32;95% 置信区间 [CI]:1.16 至 1.50)、心血管死亡(HR:1.29; 95% CI:1.11 至 1.50)、心衰再住院(HR:1.31;95% CI:1.15 至 1.49)和大出血(HR:1.71;95% CI:1.11 至 2.65)。修订前和修订后利率之间的差异仅限于东欧并由东欧驱动。该方案修订并未改变利伐沙班对主要终点(p 相互作用 = 0.36)或次要终点的中性作用。结论 在利伐沙班治疗心力衰竭的全球事件驱动试验中,要求纳入更高的 NP 会增加事件发生率,从而允许试验提前完成,但不会改变治疗效果。这些数据为未来的心力衰竭试验提供了有关基于 NP 的纳入标准对患者特征和事件发生率的预期影响的信息。 (COMMANDER HF [一项评估利伐沙班降低失代偿性心力衰竭发作后患有心力衰竭和冠状动脉疾病的参与者死亡、心肌梗塞或中风风险的有效性和安全性的研究] NCT01877915)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号