当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and docking studies of novel benzopyrone derivatives as anticonvulsants.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.bioorg.2020.103738 Walaa Hamada Abd-Allah 1 , Essam Eldin A Osman 2 , Mostafa Abd-El-Mohsen Anwar 1 , Hanan Naeim Attia 3 , Samir M El Moghazy 2
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.bioorg.2020.103738 Walaa Hamada Abd-Allah 1 , Essam Eldin A Osman 2 , Mostafa Abd-El-Mohsen Anwar 1 , Hanan Naeim Attia 3 , Samir M El Moghazy 2
Affiliation
|
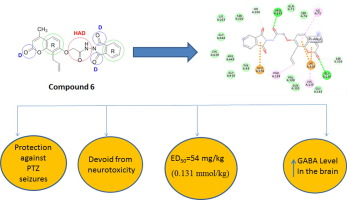
A series of coumarin derivatives 6-8, 9a-h, 11 and 13a, b -16a, b was synthesized and screened for their anticonvulsant profile. Screening of these analogues using the 'gold standard methods' revealed variable anticonvulsant potential with remarkable effects observed particularly in chemically-induced seizure test. Compounds 6, 7, 13b disclosed the highest potency among the series with 100% protection against scPTZ. Quantification study confirmed that compound 6 (ED50 0.238 mmol/kg) was the most active congener in the scPTZ model and was approximately 1.5 folds more potent than ethosuximide as reference drug Meanwhile, in the MES test, candidate drugs exhibited mild to moderate anticonvulsant efficacy, the highest of which was compound 14a, imparting 50% protection at 2.1 mmol/kg, followed by other compounds with activity ranging from 14 to 33%, as compared to diphenylhydantoin. Additionally, all candidate compounds were screened for acute neurotoxicity using the rotarod method to identify motor impairment, where almost all compounds passed the test. Further neurochemical investigation was performed to unravel the effect of the most active compound (6) on GABA level in mouse brain, where a significant elevation was evident by 4 and 1.4 folds with respect to that of the control and reference groups at p < 0.05. Molecular modeling study using Discovery Studio program was performed, where compound 6 exhibited good binding interaction with γ-aminobutyric acid aminotransferase (GABA-AT) enzyme and this was consistent with the attained experimental results.
中文翻译:

新型苯并吡喃酮衍生物作为抗惊厥药的设计,合成和对接研究。
合成了一系列香豆素衍生物6-8、9a-h,11和13a,b -16a,b,并筛选了它们的抗惊厥特性。使用“黄金标准方法”筛选这些类似物显示出可变的抗惊厥潜力,尤其在化学诱导的癫痫发作试验中观察到了显着效果。化合物6、7、13b公开了该系列中效力最高的化合物,具有针对scPTZ的100%保护。定量研究证实,化合物6(ED50为0.238 mmol / kg)是scPTZ模型中最活跃的同类物,并且比作为参考药物的ethosuximide强约1.5倍。同时,在MES测试中,候选药物显示出轻度至中度的抗惊厥功效其中最高的是化合物14a,在2.1 mmol / kg时可提供50%的保护,其次是与二苯乙内酰脲相比,具有14%至33%活性的其他化合物。此外,使用旋转仪方法筛选所有候选化合物的急性神经毒性,以识别运动障碍,几乎所有化合物都通过了测试。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。使用rotarod方法筛选所有候选化合物的急性神经毒性,以识别运动障碍,几乎所有化合物都通过了测试。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。使用rotarod方法筛选所有候选化合物的急性神经毒性,以识别运动障碍,几乎所有化合物都通过了测试。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。
更新日期:2020-03-12
中文翻译:

新型苯并吡喃酮衍生物作为抗惊厥药的设计,合成和对接研究。
合成了一系列香豆素衍生物6-8、9a-h,11和13a,b -16a,b,并筛选了它们的抗惊厥特性。使用“黄金标准方法”筛选这些类似物显示出可变的抗惊厥潜力,尤其在化学诱导的癫痫发作试验中观察到了显着效果。化合物6、7、13b公开了该系列中效力最高的化合物,具有针对scPTZ的100%保护。定量研究证实,化合物6(ED50为0.238 mmol / kg)是scPTZ模型中最活跃的同类物,并且比作为参考药物的ethosuximide强约1.5倍。同时,在MES测试中,候选药物显示出轻度至中度的抗惊厥功效其中最高的是化合物14a,在2.1 mmol / kg时可提供50%的保护,其次是与二苯乙内酰脲相比,具有14%至33%活性的其他化合物。此外,使用旋转仪方法筛选所有候选化合物的急性神经毒性,以识别运动障碍,几乎所有化合物都通过了测试。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。使用rotarod方法筛选所有候选化合物的急性神经毒性,以识别运动障碍,几乎所有化合物都通过了测试。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。使用rotarod方法筛选所有候选化合物的急性神经毒性,以识别运动障碍,几乎所有化合物都通过了测试。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。进行了进一步的神经化学研究,以揭示最具活性的化合物(6)对小鼠脑中GABA水平的影响,相对于对照组和参考组,在p <0.05时,其明显升高了4倍和1.4倍。使用Discovery Studio程序进行了分子建模研究,其中化合物6与γ-氨基丁酸氨基转移酶(GABA-AT)酶表现出良好的结合相互作用,这与获得的实验结果一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号