当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid-Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jmb.2020.03.004 W Michael Babinchak 1 , Witold K Surewicz 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jmb.2020.03.004 W Michael Babinchak 1 , Witold K Surewicz 1
Affiliation
|
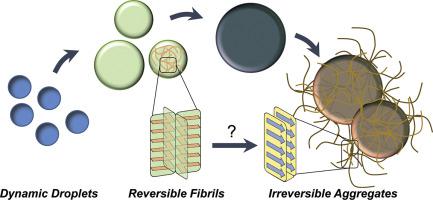
Liquid-liquid phase separation (LLPS) of proteins underlies the formation of membrane-less organelles. While it has been recognized for some time that these organelles are of key importance for normal cellular functions, a growing number of recent observations indicate that LLPS may also play a role in disease. In particular, numerous proteins that form toxic aggregates in neurodegenerative diseases, such as amyotrophic lateral sclerosis, frontotemporal lobar degeneration, and Alzheimer's disease, were found to be highly prone to phase separation, suggesting that there might be a strong link between LLPS and the pathogenic process in these disorders. This review aims to assess the molecular basis of this link through exploration of the intermolecular interactions that underlie LLPS and aggregation and the underlying mechanisms facilitating maturation of liquid droplets into more stable assemblies, including so-called labile fibrils, hydrogels, and pathological amyloids. Recent insights into the structural basis of labile fibrils and potential mechanisms by which these relatively unstable structures could transition into more stable pathogenic amyloids are also discussed. Finally, this review explores how the environment of liquid droplets could modulate protein aggregation by altering kinetics of protein self-association, affecting folding of protein monomers, or changing aggregation pathways.
中文翻译:

液-液相分离及其在病理性蛋白质聚集中的机制作用。
蛋白质的液-液相分离 (LLPS) 是形成无膜细胞器的基础。虽然一段时间以来人们已经认识到这些细胞器对正常细胞功能至关重要,但最近越来越多的观察表明 LLPS 也可能在疾病中发挥作用。特别是,发现许多在神经退行性疾病(如肌萎缩侧索硬化、额颞叶变性和阿尔茨海默病)中形成有毒聚集体的蛋白质极易发生相分离,这表明 LLPS 与致病性之间可能存在密切联系。这些疾病的过程。本综述旨在通过探索构成 LLPS 和聚集的分子间相互作用以及促进液滴成熟为更稳定的组件(包括所谓的不稳定原纤维、水凝胶和病理性淀粉样蛋白)的潜在机制来评估这种联系的分子基础。还讨论了对不稳定原纤维的结构基础和这些相对不稳定的结构可以转变为更稳定的致病性淀粉样蛋白的潜在机制的最新见解。最后,这篇综述探讨了液滴环境如何通过改变蛋白质自缔合动力学、影响蛋白质单体折叠或改变聚集途径来调节蛋白质聚集。和病理性淀粉样蛋白。还讨论了对不稳定原纤维的结构基础和这些相对不稳定的结构可以转变为更稳定的致病性淀粉样蛋白的潜在机制的最新见解。最后,这篇综述探讨了液滴环境如何通过改变蛋白质自缔合动力学、影响蛋白质单体折叠或改变聚集途径来调节蛋白质聚集。和病理性淀粉样蛋白。还讨论了对不稳定原纤维的结构基础和这些相对不稳定的结构可以转变为更稳定的致病性淀粉样蛋白的潜在机制的最新见解。最后,这篇综述探讨了液滴环境如何通过改变蛋白质自缔合动力学、影响蛋白质单体折叠或改变聚集途径来调节蛋白质聚集。
更新日期:2020-03-10
中文翻译:

液-液相分离及其在病理性蛋白质聚集中的机制作用。
蛋白质的液-液相分离 (LLPS) 是形成无膜细胞器的基础。虽然一段时间以来人们已经认识到这些细胞器对正常细胞功能至关重要,但最近越来越多的观察表明 LLPS 也可能在疾病中发挥作用。特别是,发现许多在神经退行性疾病(如肌萎缩侧索硬化、额颞叶变性和阿尔茨海默病)中形成有毒聚集体的蛋白质极易发生相分离,这表明 LLPS 与致病性之间可能存在密切联系。这些疾病的过程。本综述旨在通过探索构成 LLPS 和聚集的分子间相互作用以及促进液滴成熟为更稳定的组件(包括所谓的不稳定原纤维、水凝胶和病理性淀粉样蛋白)的潜在机制来评估这种联系的分子基础。还讨论了对不稳定原纤维的结构基础和这些相对不稳定的结构可以转变为更稳定的致病性淀粉样蛋白的潜在机制的最新见解。最后,这篇综述探讨了液滴环境如何通过改变蛋白质自缔合动力学、影响蛋白质单体折叠或改变聚集途径来调节蛋白质聚集。和病理性淀粉样蛋白。还讨论了对不稳定原纤维的结构基础和这些相对不稳定的结构可以转变为更稳定的致病性淀粉样蛋白的潜在机制的最新见解。最后,这篇综述探讨了液滴环境如何通过改变蛋白质自缔合动力学、影响蛋白质单体折叠或改变聚集途径来调节蛋白质聚集。和病理性淀粉样蛋白。还讨论了对不稳定原纤维的结构基础和这些相对不稳定的结构可以转变为更稳定的致病性淀粉样蛋白的潜在机制的最新见解。最后,这篇综述探讨了液滴环境如何通过改变蛋白质自缔合动力学、影响蛋白质单体折叠或改变聚集途径来调节蛋白质聚集。











































 京公网安备 11010802027423号
京公网安备 11010802027423号