当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteomic Analyses Identify a Novel Role for EZH2 in the Initiation of Cancer Cell Drug Tolerance.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-03-11 , DOI: 10.1021/acs.jproteome.9b00773 Victoria Pham , Robert Pitti , Charles A. Tindell , Tommy K. Cheung , Alexandre Masselot , Jean-Phillipe Stephan , Gulfem D. Guler , Catherine Wilson , Jennie Lill , David Arnott , Marie Classon
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-03-11 , DOI: 10.1021/acs.jproteome.9b00773 Victoria Pham , Robert Pitti , Charles A. Tindell , Tommy K. Cheung , Alexandre Masselot , Jean-Phillipe Stephan , Gulfem D. Guler , Catherine Wilson , Jennie Lill , David Arnott , Marie Classon
|
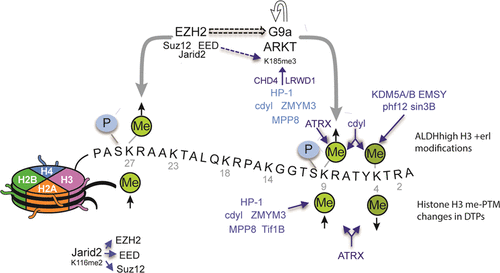
Acquisition of drug resistance remains a chief impediment to successful cancer therapy, and we previously described a transient drug-tolerant cancer cell population (DTPs) whose survival is in part dependent on the activities of the histone methyltransferases G9a/EHMT2 and EZH2, the latter being the catalytic component of the polycomb repressive complex 2 (PRC2). Here, we apply multiple proteomic techniques to better understand the role of these histone methyltransferases (HMTs) in the establishment of the DTP state. Proteome-wide comparisons of lysine methylation patterns reveal that DTPs display an increase in methylation on K116 of PRC member Jarid2, an event that helps stabilize and recruit PRC2 to chromatin. We also find that EZH2, in addition to methylating histone H3K27, also can methylate G9a at K185, and that methylated G9a better recruits repressive complexes to chromatin. These complexes are similar to complexes recruited by histone H3 methylated at K9. Finally, a detailed histone post-translational modification (PTM) analysis shows that EZH2, either directly or through its ability to methylate G9a, alters H3K9 methylation in the context of H3 serine 10 phosphorylation, primarily in a cancer cell subpopulation that serves as DTP precursors. We also show that combinations of histone PTMs recruit a different set of complexes to chromatin, shedding light on the temporal mechanisms that contribute to drug tolerance.
中文翻译:

蛋白质组学分析确定了EZH2在癌细胞耐受性启动中的新作用。
获得耐药性仍然是成功进行癌症治疗的主要障碍,我们之前已经描述了短暂的耐药性癌细胞群(DTP),其存活部分取决于组蛋白甲基转移酶G9a / EHMT2和EZH2的活性,后者是聚梳阻抑复合物2(PRC2)的催化成分。在这里,我们应用多种蛋白质组学技术来更好地理解这些组蛋白甲基转移酶(HMT)在DTP状态建立中的作用。蛋白质组范围内赖氨酸甲基化模式的比较显示,DTP在PRC成员Jarid2的K116上显示甲基化增加,该事件有助于稳定和募集PRC2至染色质。我们还发现EZH2除了可以将组蛋白H3K27甲基化外,还可以将K185处的G9a甲基化,甲基化的G9a可以更好地募集抑制复合物至染色质。这些复合物类似于由在K9处甲基化的组蛋白H3募集的复合物。最后,详细的组蛋白翻译后修饰(PTM)分析表明,EZH2直接或通过其甲基化G9a的能力,在H3丝氨酸10磷酸化的情况下,主要在充当DTP前体的癌细胞亚群中改变了H3K9甲基化。 。我们还显示,组蛋白PTM的组合招募到染色质的一组不同的复合物,从而减轻了造成药物耐受性的时间机制。直接或通过其甲基化G9a的能力,在H3丝氨酸10磷酸化的背景下,主要是在充当DTP前体的癌细胞亚群中,改变H3K9甲基化。我们还显示,组蛋白PTM的组合招募到染色质的一组不同的复合物,从而减轻了造成药物耐受性的时间机制。直接或通过其甲基化G9a的能力,在H3丝氨酸10磷酸化的背景下,主要是在充当DTP前体的癌细胞亚群中,改变H3K9甲基化。我们还显示,组蛋白PTM的组合招募到染色质的一组不同的复合物,从而减轻了造成药物耐受性的时间机制。
更新日期:2020-04-24
中文翻译:

蛋白质组学分析确定了EZH2在癌细胞耐受性启动中的新作用。
获得耐药性仍然是成功进行癌症治疗的主要障碍,我们之前已经描述了短暂的耐药性癌细胞群(DTP),其存活部分取决于组蛋白甲基转移酶G9a / EHMT2和EZH2的活性,后者是聚梳阻抑复合物2(PRC2)的催化成分。在这里,我们应用多种蛋白质组学技术来更好地理解这些组蛋白甲基转移酶(HMT)在DTP状态建立中的作用。蛋白质组范围内赖氨酸甲基化模式的比较显示,DTP在PRC成员Jarid2的K116上显示甲基化增加,该事件有助于稳定和募集PRC2至染色质。我们还发现EZH2除了可以将组蛋白H3K27甲基化外,还可以将K185处的G9a甲基化,甲基化的G9a可以更好地募集抑制复合物至染色质。这些复合物类似于由在K9处甲基化的组蛋白H3募集的复合物。最后,详细的组蛋白翻译后修饰(PTM)分析表明,EZH2直接或通过其甲基化G9a的能力,在H3丝氨酸10磷酸化的情况下,主要在充当DTP前体的癌细胞亚群中改变了H3K9甲基化。 。我们还显示,组蛋白PTM的组合招募到染色质的一组不同的复合物,从而减轻了造成药物耐受性的时间机制。直接或通过其甲基化G9a的能力,在H3丝氨酸10磷酸化的背景下,主要是在充当DTP前体的癌细胞亚群中,改变H3K9甲基化。我们还显示,组蛋白PTM的组合招募到染色质的一组不同的复合物,从而减轻了造成药物耐受性的时间机制。直接或通过其甲基化G9a的能力,在H3丝氨酸10磷酸化的背景下,主要是在充当DTP前体的癌细胞亚群中,改变H3K9甲基化。我们还显示,组蛋白PTM的组合招募到染色质的一组不同的复合物,从而减轻了造成药物耐受性的时间机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号