Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microcalorimetric Analysis of the Adsorption of Lysozyme and Cytochrome c onto Cation-Exchange Chromatography Resins: Influence of Temperature on Retention.
Langmuir ( IF 3.7 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.langmuir.0c00197 Joao C Simoes-Cardoso 1 , Hiroshi Kojo 1 , Noriko Yoshimoto 1 , Shuichi Yamamoto 1
Langmuir ( IF 3.7 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.langmuir.0c00197 Joao C Simoes-Cardoso 1 , Hiroshi Kojo 1 , Noriko Yoshimoto 1 , Shuichi Yamamoto 1
Affiliation
|
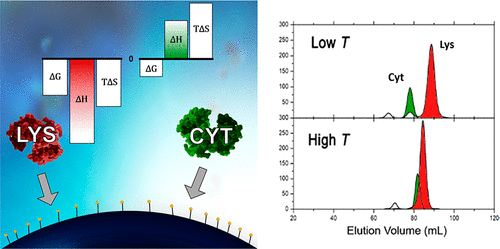
We studied the adsorption mechanism of two basic proteins, equine cytochrome c (Cyt) and chicken egg-white lysozyme (Lys), adsorbing onto negatively charged chromatography surfaces. In liquid chromatography, the retention volume of Lys was larger than that of Cyt on negatively charged ion-exchange resins. When the temperature increased, the retention volume of Cyt increased, whereas that of Lys clearly decreased. Both Lys and Cyt share similar physical characteristics, so the opposite behavior with increasing temperatures was surprising, indicating a more complex mechanism of adsorption may be involved. We analyzed the adsorption of these proteins by using isothermal titration calorimetry (ITC). The change in adsorption enthalpy determined by ITC allowed the understanding of the reason for and underlying driving forces of protein adsorption that resulted in this opposite behavior. Large exothermic enthalpies of adsorption were observed for Lys (−43.95 kJ/mol), and Lys adsorption was found to be enthalpically driven. On the other hand, endothermic enthalpies were dominant for Cyt adsorption (32.41 kJ/mol), which was entropically driven. These results indicate that dehydration and release of counterions play a more important role in Cyt adsorption and ionic interaction and hydrogen bridges are more significant in Lys adsorption. Understanding of the adsorption mechanism of proteins onto chromatography resins is essential for modeling and developing new, efficient chromatographic processes.
中文翻译:

微量热法分析溶菌酶和细胞色素c在阳离子交换色谱树脂上的吸附:温度对保留率的影响。
我们研究了两种基本蛋白,马细胞色素c(Cyt)和鸡卵清溶菌酶(Lys)的吸附机理,吸附到带负电荷的色谱表面。在液相色谱中,在带负电的离子交换树脂上,Lys的保留体积大于Cyt的保留体积。当温度升高时,Cyt的保留体积增加,而Lys的保留体积明显减少。Lys和Cyt都具有相似的物理特性,因此随着温度的升高,其相反的行为令人惊讶,这表明可能涉及更复杂的吸附机理。我们通过使用等温滴定量热法(ITC)分析了这些蛋白质的吸附。由ITC确定的吸附焓的变化可以理解导致这种相反行为的蛋白质吸附的原因和潜在的驱动力。对于Lys(-43.95 kJ / mol)观察到了较大的放热吸附焓,发现Lys的吸附是焓驱动的。另一方面,吸热焓是Cyt吸附的主要因素(32.41 kJ / mol),这是由熵驱动的。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。对于Lys(-43.95 kJ / mol)观察到了较大的放热吸附焓,发现Lys的吸附是焓驱动的。另一方面,吸热焓是Cyt吸附的主要因素(32.41 kJ / mol),这是由熵驱动的。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,而氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。对于Lys(-43.95 kJ / mol)观察到了较大的放热吸附焓,发现Lys的吸附是焓驱动的。另一方面,吸热焓是Cyt吸附的主要因素(32.41 kJ / mol),这是由熵驱动的。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。
更新日期:2020-03-24
中文翻译:

微量热法分析溶菌酶和细胞色素c在阳离子交换色谱树脂上的吸附:温度对保留率的影响。
我们研究了两种基本蛋白,马细胞色素c(Cyt)和鸡卵清溶菌酶(Lys)的吸附机理,吸附到带负电荷的色谱表面。在液相色谱中,在带负电的离子交换树脂上,Lys的保留体积大于Cyt的保留体积。当温度升高时,Cyt的保留体积增加,而Lys的保留体积明显减少。Lys和Cyt都具有相似的物理特性,因此随着温度的升高,其相反的行为令人惊讶,这表明可能涉及更复杂的吸附机理。我们通过使用等温滴定量热法(ITC)分析了这些蛋白质的吸附。由ITC确定的吸附焓的变化可以理解导致这种相反行为的蛋白质吸附的原因和潜在的驱动力。对于Lys(-43.95 kJ / mol)观察到了较大的放热吸附焓,发现Lys的吸附是焓驱动的。另一方面,吸热焓是Cyt吸附的主要因素(32.41 kJ / mol),这是由熵驱动的。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。对于Lys(-43.95 kJ / mol)观察到了较大的放热吸附焓,发现Lys的吸附是焓驱动的。另一方面,吸热焓是Cyt吸附的主要因素(32.41 kJ / mol),这是由熵驱动的。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,而氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。对于Lys(-43.95 kJ / mol)观察到了较大的放热吸附焓,发现Lys的吸附是焓驱动的。另一方面,吸热焓是Cyt吸附的主要因素(32.41 kJ / mol),这是由熵驱动的。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。这些结果表明,抗衡离子的脱水和释放在Cyt吸附和离子相互作用中起更重要的作用,氢桥在Lys吸附中更重要。了解蛋白质在色谱树脂上的吸附机理对于建模和开发新的有效色谱方法至关重要。









































 京公网安备 11010802027423号
京公网安备 11010802027423号