当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthesis of Amino Acid Derived α-Pyrones by an NRPS-NRPKS Hybrid Megasynthetase in Fungi.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-11 , DOI: 10.1021/acs.jnatprod.9b00989 Yang Hai , Arthur Huang , Yi Tang
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-11 , DOI: 10.1021/acs.jnatprod.9b00989 Yang Hai , Arthur Huang , Yi Tang
|
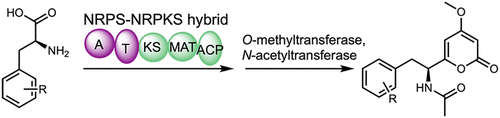
A nonribosomal peptide synthetase (NRPS)-nonreducing polyketide synthase (NRPKS) hybrid enzyme (AnATPKS) from Aspergillus niger was shown to produce amino acid derived α-pyrone natural products (pyrophen and campyrone B). Biochemical characterization of the NRPS module in vitro reveals that the adenylation domain is promiscuous toward a variety of substituted phenylalanine analogues. Using precursor feeding and heterologous expression of AnATPKS and an associated O-methyltransferase (AnOMT), we were able to access a library of substituted pyrophen analogues. Our study paves the way for future combinatorial biosynthesis of diverse α-pyrone natural products using NRPS-NRPKS hybrids.
中文翻译:

在真菌中通过 NRPS-NRPKS 杂合超级合成酶生物合成氨基酸衍生的 α-吡喃酮。
来自黑曲霉的非核糖体肽合成酶 (NRPS)-非还原性聚酮化合物合酶 (NRPKS) 杂合酶 (AnATPKS) 被证明可以产生氨基酸衍生的 α-吡喃酮天然产物(焦酚和樟脑酮 B)。NRPS 模块的体外生化表征表明,腺苷酸化结构域与各种取代的苯丙氨酸类似物混杂。使用 AnATPKS 和相关 O-甲基转移酶 (AnOMT) 的前体喂养和异源表达,我们能够访问取代的焦酚类似物库。我们的研究为未来使用 NRPS-NRPKS 杂种组合生物合成多种 α-吡喃酮天然产物铺平了道路。
更新日期:2020-04-24
中文翻译:

在真菌中通过 NRPS-NRPKS 杂合超级合成酶生物合成氨基酸衍生的 α-吡喃酮。
来自黑曲霉的非核糖体肽合成酶 (NRPS)-非还原性聚酮化合物合酶 (NRPKS) 杂合酶 (AnATPKS) 被证明可以产生氨基酸衍生的 α-吡喃酮天然产物(焦酚和樟脑酮 B)。NRPS 模块的体外生化表征表明,腺苷酸化结构域与各种取代的苯丙氨酸类似物混杂。使用 AnATPKS 和相关 O-甲基转移酶 (AnOMT) 的前体喂养和异源表达,我们能够访问取代的焦酚类似物库。我们的研究为未来使用 NRPS-NRPKS 杂种组合生物合成多种 α-吡喃酮天然产物铺平了道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号