当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-Dimensional Chemical Mapping of a Single Protein in the Hydrated State with Atom Probe Tomography.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.analchem.9b05668 Shi Qiu 1 , Changxi Zheng 2, 3 , Vivek Garg 1, 4 , Yu Chen 5 , Gediminas Gervinskas 6 , Jian Li 7, 8 , Michelle A Dunstone 8, 9 , Ross K W Marceau 10 , Jing Fu 1, 9
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.analchem.9b05668 Shi Qiu 1 , Changxi Zheng 2, 3 , Vivek Garg 1, 4 , Yu Chen 5 , Gediminas Gervinskas 6 , Jian Li 7, 8 , Michelle A Dunstone 8, 9 , Ross K W Marceau 10 , Jing Fu 1, 9
Affiliation
|
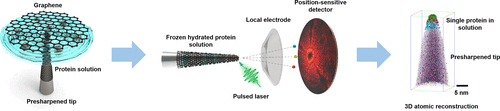
Unravelling the three-dimensional structures and compositions of biological macromolecules sheds light on their functions and also contributes to the design of future biochemical compounds and processes. Atom probe tomography (APT) is demonstrated in this research as a new and effective approach to explore the structure and chemical composition of a single protein in the hydrated state. By introducing graphene encapsulation, proteins in solution can be immobilized on a metal specimen tip, with an end radius in the range of 50 nm to allow field ionization and evaporation. Using a ferritin particle as an example, analysis of the mass spectrum and reconstructed 3D chemical maps at near-atomic resolution acquired from APT reveals the core consisting of iron and iron oxides, the peptide shell containing amino acids, and the interior interface between the iron core and the peptide shell. The quantitative distribution and proportion of iron isotopes from a single ferritin core have been determined for the first time, as well as identification of the possible sites of amino acids inside the protein shell. The complete experimental protocol is straightforward and lays a foundation for future exploration of various macromolecules in a controlled environment.
中文翻译:

原子探针层析成像在水合状态下单个蛋白质的三维化学图谱。
揭示生物大分子的三维结构和组成揭示了它们的功能,也有助于未来生物化学化合物和工艺的设计。在这项研究中,原子探针层析成像(APT)被证明是探索水合状态下单个蛋白质的结构和化学组成的一种新的有效方法。通过引入石墨烯封装,可以将溶液中的蛋白质固定在金属样品尖端上,其末端半径在50 nm范围内,以实现电场电离和蒸发。以铁蛋白颗粒为例,从APT获得的质谱分析和以近原子分辨率重建的3D化学图谱揭示了由铁和铁氧化物组成的核心,其中的肽壳含有氨基酸,以及铁核和肽壳之间的内部界面。首次确定了来自单个铁蛋白核心的铁同位素的定量分布和比例,以及鉴定了蛋白质壳内部氨基酸的可能位点。完整的实验方案非常简单,为将来在受控环境中探索各种大分子奠定了基础。
更新日期:2020-03-26
中文翻译:

原子探针层析成像在水合状态下单个蛋白质的三维化学图谱。
揭示生物大分子的三维结构和组成揭示了它们的功能,也有助于未来生物化学化合物和工艺的设计。在这项研究中,原子探针层析成像(APT)被证明是探索水合状态下单个蛋白质的结构和化学组成的一种新的有效方法。通过引入石墨烯封装,可以将溶液中的蛋白质固定在金属样品尖端上,其末端半径在50 nm范围内,以实现电场电离和蒸发。以铁蛋白颗粒为例,从APT获得的质谱分析和以近原子分辨率重建的3D化学图谱揭示了由铁和铁氧化物组成的核心,其中的肽壳含有氨基酸,以及铁核和肽壳之间的内部界面。首次确定了来自单个铁蛋白核心的铁同位素的定量分布和比例,以及鉴定了蛋白质壳内部氨基酸的可能位点。完整的实验方案非常简单,为将来在受控环境中探索各种大分子奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号