当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stretching the P-C Bond. Variations on Carbenes and Phosphanes.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jpca.0c00641 Dániel Buzsáki 1 , Zsolt Kelemen 1 , László Nyulászi 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.jpca.0c00641 Dániel Buzsáki 1 , Zsolt Kelemen 1 , László Nyulászi 1, 2
Affiliation
|
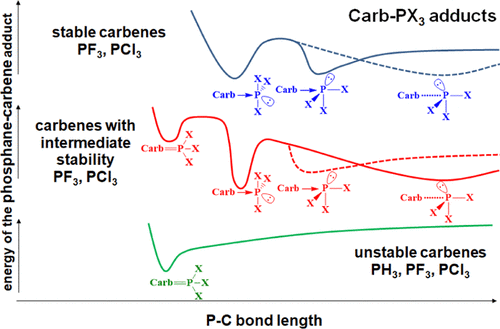
The stability and the structure of adducts formed between four substituted phosphanes (PX3, X:H, F, Cl, and NMe2) and 11 different carbenes have been investigated by DFT calculations. In most cases, the structure of the adducts depends strongly on the stability of the carbene itself, exhibiting a linear correlation with the increasing dissociation energy of the adduct. Carbenes of low stability form phosphorus ylides (F), which can be described as phosphane → carbene adducts supported with some back-bonding. The most stable carbenes, which have high energy lone pair, do not form stable F-type structures but carbene → phosphane adducts (E-type structure), utilizing the low-lying lowest unoccupied molecular orbital (LUMO) of the phosphane (with electronegative substituents), benefiting also from the carbene–pnictogen interaction. Especially noteworthy is the case of PCl3, which has an extremely low energy LUMO in its T-shaped form. Although this PCl3 structure is a transition state of rather high energy, the large stabilization energy of the complex makes this carbene–phosphane adduct stable. Most interestingly, in case of carbenes with medium stability both F- and E-type structures could be optimized, giving rise to bond-stretch isomerism. Likewise, for phosphorus ylides (F), the stability of the adducts G formed from carbenes with hypovalent phosphorus (PX—phosphinidene) is in a linear relationship with the stabilization of the carbene. Adducts of carbenes with hypervalent phosphorus (PX5) are the most stable when X is electronegative, and the carbene is highly nucleophilic.
中文翻译:

延长 PC 债券。卡宾和磷烷的变体。
通过DFT计算研究了四种取代膦(PX 3、X:H、F、Cl和NMe 2 )和11种不同卡宾之间形成的加合物的稳定性和结构。在大多数情况下,加合物的结构很大程度上取决于卡宾本身的稳定性,与加合物解离能的增加呈线性相关。低稳定性的卡宾形成磷叶立德(F),其可以被描述为由一些背键支撑的磷烷→卡宾加合物。最稳定的卡宾具有高能孤对电子,不会形成稳定的F型结构,而是利用膦的低位最低未占分子轨道(LUMO)形成卡宾→膦加合物(E型结构)(具有负电性)取代基),也受益于卡宾-磷元素相互作用。尤其值得注意的是 PCl 3的情况,其 T 形形式的 LUMO 能量极低。虽然这种PCl 3结构是相当高能量的过渡态,但配合物的大稳定能使得这种卡宾-膦加合物稳定。最有趣的是,对于中等稳定性的卡宾,F 型和E型结构都可以优化,从而产生键拉伸异构现象。同样,对于磷叶立德(F ),由卡宾与低价磷(PX-次膦亚基)形成的加合物G的稳定性与卡宾的稳定性呈线性关系。当X带负电时,卡宾与高价磷(PX 5 )的加合物最稳定,并且卡宾具有高度亲核性。
更新日期:2020-03-24
中文翻译:

延长 PC 债券。卡宾和磷烷的变体。
通过DFT计算研究了四种取代膦(PX 3、X:H、F、Cl和NMe 2 )和11种不同卡宾之间形成的加合物的稳定性和结构。在大多数情况下,加合物的结构很大程度上取决于卡宾本身的稳定性,与加合物解离能的增加呈线性相关。低稳定性的卡宾形成磷叶立德(F),其可以被描述为由一些背键支撑的磷烷→卡宾加合物。最稳定的卡宾具有高能孤对电子,不会形成稳定的F型结构,而是利用膦的低位最低未占分子轨道(LUMO)形成卡宾→膦加合物(E型结构)(具有负电性)取代基),也受益于卡宾-磷元素相互作用。尤其值得注意的是 PCl 3的情况,其 T 形形式的 LUMO 能量极低。虽然这种PCl 3结构是相当高能量的过渡态,但配合物的大稳定能使得这种卡宾-膦加合物稳定。最有趣的是,对于中等稳定性的卡宾,F 型和E型结构都可以优化,从而产生键拉伸异构现象。同样,对于磷叶立德(F ),由卡宾与低价磷(PX-次膦亚基)形成的加合物G的稳定性与卡宾的稳定性呈线性关系。当X带负电时,卡宾与高价磷(PX 5 )的加合物最稳定,并且卡宾具有高度亲核性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号