当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Next-generation sequencing for BCR-ABL1 kinase domain mutations in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A position paper.
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-03-10 , DOI: 10.1002/cam4.2946 Simona Soverini 1 , Francesco Albano 2 , Renato Bassan 3 , Francesco Fabbiano 4 , Felicetto Ferrara 5 , Robin Foà 6 , Attilio Olivieri 7 , Alessandro Rambaldi 8 , Giuseppe Rossi 9 , Simona Sica 10, 11 , Giorgina Specchia 2 , Adriano Venditti 12 , Giovanni Barosi 13 , Fabrizio Pane 14
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-03-10 , DOI: 10.1002/cam4.2946 Simona Soverini 1 , Francesco Albano 2 , Renato Bassan 3 , Francesco Fabbiano 4 , Felicetto Ferrara 5 , Robin Foà 6 , Attilio Olivieri 7 , Alessandro Rambaldi 8 , Giuseppe Rossi 9 , Simona Sica 10, 11 , Giorgina Specchia 2 , Adriano Venditti 12 , Giovanni Barosi 13 , Fabrizio Pane 14
Affiliation
|
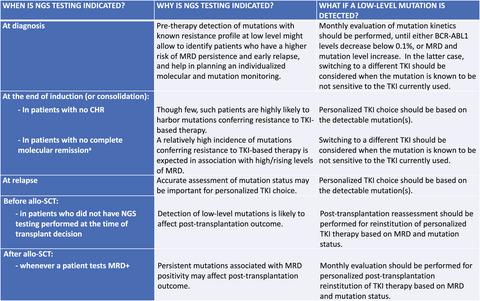
Emergence of clones carrying point mutations in the BCR-ABL1 kinase domain (KD) is a common mechanism of resistance to tyrosine kinase inhibitor (TKI)-based therapies in Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Sanger sequencing (SS) is the most frequently used method for diagnostic BCR-ABL1 KD mutation screening, but it has some limitations-it is poorly sensitive and cannot robustly identify compound mutations. Next-generation sequencing (NGS) may overcome these problems. NSG is increasingly available and has the potential to become the method of choice for diagnostic BCR-ABL1 KD mutation screening. A group discussion within an ad hoc constituted Panel of Experts has produced a series of consensus-based statements on the potential value of NGS testing before and during first-line TKI-based treatment, in relapsed/refractory cases, before and after allo-stem cell transplantation, and on how NGS results may impact on therapeutic decisions. A set of minimal technical and methodological requirements for the analysis and the reporting of results has also been defined. The proposals herein reported may be used to guide the practical use of NGS for BCR-ABL1 KD mutation testing in Ph+ ALL.
中文翻译:

费城染色体阳性急性淋巴细胞白血病成年患者中BCR-ABL1激酶结构域突变的下一代测序:立场文件。
在费城染色体阳性(Ph +)急性淋巴细胞白血病(ALL)中,BCR-ABL1激酶域(KD)中带有点突变的克隆的出现是对基于酪氨酸激酶抑制剂(TKI)疗法的耐药性的常见机制。Sanger测序(SS)是诊断BCR-ABL1 KD突变筛查最常用的方法,但是它有一些局限性-敏感性很差,无法可靠地鉴定化合物突变。下一代测序(NGS)可以克服这些问题。NSG越来越多,并且有可能成为诊断BCR-ABL1 KD突变筛查的首选方法。在特设的专家小组中进行的小组讨论针对基于TKI的一线治疗之前和期间,NGS检测的潜在价值提出了一系列基于共识的陈述,在复发/难治性病例中,同种异体干细胞移植之前和之后,以及NGS的结果如何可能影响治疗决策。还定义了一组用于分析和报告结果的最低技术和方法要求。本文报道的建议可用于指导NGS在Ph + ALL中用于BCR-ABL1 KD突变测试的实际应用。
更新日期:2020-03-10
中文翻译:

费城染色体阳性急性淋巴细胞白血病成年患者中BCR-ABL1激酶结构域突变的下一代测序:立场文件。
在费城染色体阳性(Ph +)急性淋巴细胞白血病(ALL)中,BCR-ABL1激酶域(KD)中带有点突变的克隆的出现是对基于酪氨酸激酶抑制剂(TKI)疗法的耐药性的常见机制。Sanger测序(SS)是诊断BCR-ABL1 KD突变筛查最常用的方法,但是它有一些局限性-敏感性很差,无法可靠地鉴定化合物突变。下一代测序(NGS)可以克服这些问题。NSG越来越多,并且有可能成为诊断BCR-ABL1 KD突变筛查的首选方法。在特设的专家小组中进行的小组讨论针对基于TKI的一线治疗之前和期间,NGS检测的潜在价值提出了一系列基于共识的陈述,在复发/难治性病例中,同种异体干细胞移植之前和之后,以及NGS的结果如何可能影响治疗决策。还定义了一组用于分析和报告结果的最低技术和方法要求。本文报道的建议可用于指导NGS在Ph + ALL中用于BCR-ABL1 KD突变测试的实际应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号