当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Confinement of Intermediates in Blue TiO2 Nanotube Arrays Boosts Reaction Rate of Nitrogen Electrocatalysis
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-09 , DOI: 10.1002/cctc.202000006 Jianfang Zhang 1, 2 , Yujing Tian 1 , Tianyu Zhang 1 , Zhengyuan Li 1 , Xiaojie She 1 , Yucheng Wu 2 , Yan Wang 2 , Jingjie Wu 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-09 , DOI: 10.1002/cctc.202000006 Jianfang Zhang 1, 2 , Yujing Tian 1 , Tianyu Zhang 1 , Zhengyuan Li 1 , Xiaojie She 1 , Yucheng Wu 2 , Yan Wang 2 , Jingjie Wu 1
Affiliation
|
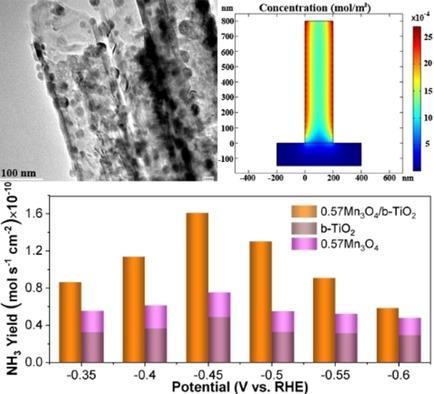
Electrocatalytic nitrogen reduction reaction (NRR) is a complementary route to the traditional Haber‐Bosch process. Boosting the reaction kinetics of electrocatalytic NRR is key to promote the NH3 yield. Herein, we demonstrate that the confinement of reactive intermediates (e. g., *N2) in highly ordered blue TiO2 nanotubes (b‐TiO2) can significantly enhance the surface reaction rate of electrocatalytic NRR on the encapsulated metal oxide catalysts. The enhancement of the reaction rate of NH3 synthesis is attributed to the increased surface coverage of intermediates inside the b‐TiO2 nanotubes. Benefiting from the confinement effect of intermediates, the b‐TiO2 nanotubes enclosing metal oxide electrodes show both higher production rate and Faradaic efficiency of NH3 than the combined ones of individual metal oxide and b‐TiO2 electrodes.
中文翻译:

蓝色TiO2纳米管阵列中中间体的限制提高了氮电催化的反应速率。
电催化氮还原反应(NRR)是传统Haber-Bosch工艺的补充途径。提高电催化NRR的反应动力学是提高NH 3收率的关键。在本文中,我们表明,反应中间体的约束(例如,* N 2)在高度有序的蓝色的TiO 2纳米管(B-的TiO 2)可以显著加强对包封的金属氧化物催化剂的电催化NRR的表面反应速率。NH 3合成反应速率的提高归因于b-TiO 2纳米管内部中间体的表面积增加。得益于中间体b‐TiO 2的封闭作用与单独的金属氧化物电极和b-TiO 2电极的组合电极相比,包围金属氧化物电极的纳米管显示出更高的生产率和NH 3的法拉第效率。
更新日期:2020-03-09
中文翻译:

蓝色TiO2纳米管阵列中中间体的限制提高了氮电催化的反应速率。
电催化氮还原反应(NRR)是传统Haber-Bosch工艺的补充途径。提高电催化NRR的反应动力学是提高NH 3收率的关键。在本文中,我们表明,反应中间体的约束(例如,* N 2)在高度有序的蓝色的TiO 2纳米管(B-的TiO 2)可以显著加强对包封的金属氧化物催化剂的电催化NRR的表面反应速率。NH 3合成反应速率的提高归因于b-TiO 2纳米管内部中间体的表面积增加。得益于中间体b‐TiO 2的封闭作用与单独的金属氧化物电极和b-TiO 2电极的组合电极相比,包围金属氧化物电极的纳米管显示出更高的生产率和NH 3的法拉第效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号