当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Arylation of Diketopyrrolopyrroles with Aryl Triflates.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-03-24 , DOI: 10.1002/asia.202000129 Krzysztof Gutkowski 1 , Kamil Skonieczny 1 , Marta Bugaj 1 , Denis Jacquemin 2 , Daniel T Gryko 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-03-24 , DOI: 10.1002/asia.202000129 Krzysztof Gutkowski 1 , Kamil Skonieczny 1 , Marta Bugaj 1 , Denis Jacquemin 2 , Daniel T Gryko 1
Affiliation
|
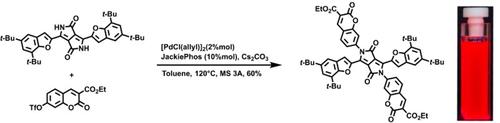
A new methodology for the double N-arylation of diketopyrrolopyrroles with aryl triflates has been developed. It is now possible to prepare diketopyrrolopyrroles bearing N-substituents derived from naphthalene, anthracene and coumarin in two steps from commercially available phenols. This represents the first time arenes lacking strong electron-withdrawing groups were inserted onto lactamic nitrogen atoms via arylation. The ability to incorporate heretofore unprecedented substituents translates to increased modulation of the resulting photophysical properties such as switching-on/off solvatofluorochromism. TD-DFT calculations have been performed to explore the nature of the relevant excited states. This new synthetic method made it possible to elucidate the influence of such substituents on the absorption and emission properties of tetraaryl substituted diketopyrrolopyrroles.
中文翻译:

二酮吡咯并吡咯与芳基三氟甲磺酸酯的N-芳基化作用。
已经开发出一种新的方法,用于二酮吡咯并吡咯与芳基三氟甲磺酸酯的双N-芳基化。现在可以用两步法从市售苯酚制备带有衍生自萘,蒽和香豆素的N-取代基的二酮吡咯并吡咯。这代表缺乏芳电子吸收基团的芳烃第一次通过芳基化作用插入内酰胺氮原子上。迄今为止引入前所未有的取代基的能力转化为增加的对所产生的光物理性质的调节,例如开/关溶剂氟发色。进行了TD-DFT计算以探索相关激发态的性质。
更新日期:2020-04-22
中文翻译:

二酮吡咯并吡咯与芳基三氟甲磺酸酯的N-芳基化作用。
已经开发出一种新的方法,用于二酮吡咯并吡咯与芳基三氟甲磺酸酯的双N-芳基化。现在可以用两步法从市售苯酚制备带有衍生自萘,蒽和香豆素的N-取代基的二酮吡咯并吡咯。这代表缺乏芳电子吸收基团的芳烃第一次通过芳基化作用插入内酰胺氮原子上。迄今为止引入前所未有的取代基的能力转化为增加的对所产生的光物理性质的调节,例如开/关溶剂氟发色。进行了TD-DFT计算以探索相关激发态的性质。









































 京公网安备 11010802027423号
京公网安备 11010802027423号