当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Modular Synthesis of Antitumor Macrolide (–)‐Lasonolide A†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-10 , DOI: 10.1002/cjoc.202000026 Lin Yang 1, 2 , Zuming Lin 1 , Kuan Zheng 1, 2 , Luyao Kong 2 , Ran Hong 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-10 , DOI: 10.1002/cjoc.202000026 Lin Yang 1, 2 , Zuming Lin 1 , Kuan Zheng 1, 2 , Luyao Kong 2 , Ran Hong 1
Affiliation
|
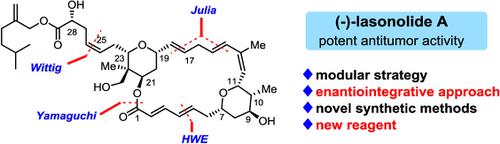
Lasonolide A was identified as a potent antitumor macrolide towards various cancer cell lines. The two tetrahydropyrans bearing multiple stereogenic centers as well as the polyene linkage attracted a dozen synthetic research groups to launch the total synthesis. Based on the synthetic methods developed in our group, namely, the hydroboration of allene and its subsequent allylation as well as the iterative hydroboration of allene and oxidation, the polyol subunits were efficiently constructed and then integrated into the final target. A new Julia olefination reagent, double‐headed sulfone, was designed to promote the rapid coupling of two aldehydes bearing multiple functional groups to secure the whole carbon framework. Another highlight of our approach is the application of a traceless protecting group, 9‐BBN (9‐borabicyclo[3.3.1]nonane), to hide the secondary alcohol for debenzylation, and for the first time, to mask the carboxylic acid for Julia olefination under strong basic conditions.
中文翻译:

抗肿瘤大环内酯(–)-Lasonolide A†的模块合成
Lasonolide A被确定为针对各种癌细胞的有效抗肿瘤大环内酯类药物。带有多个立体中心和多烯键的两个四氢吡喃吸引了十几个合成研究小组发起了全合成研究。基于我们小组开发的合成方法,即艾伦的硼氢化及其随后的烯丙基化,以及艾伦的迭代氢硼化和氧化,可以有效地构建多元醇亚基,然后将其整合到最终目标中。设计了一种新的Julia烯烃化试剂双头砜,以促进带有多个官能团的两个醛的快速偶联,从而确保整个碳骨架。我们方法的另一个亮点是应用了无痕保护基9-BBN(9-硼环[3.3.1]壬烷),
更新日期:2020-03-10
中文翻译:

抗肿瘤大环内酯(–)-Lasonolide A†的模块合成
Lasonolide A被确定为针对各种癌细胞的有效抗肿瘤大环内酯类药物。带有多个立体中心和多烯键的两个四氢吡喃吸引了十几个合成研究小组发起了全合成研究。基于我们小组开发的合成方法,即艾伦的硼氢化及其随后的烯丙基化,以及艾伦的迭代氢硼化和氧化,可以有效地构建多元醇亚基,然后将其整合到最终目标中。设计了一种新的Julia烯烃化试剂双头砜,以促进带有多个官能团的两个醛的快速偶联,从而确保整个碳骨架。我们方法的另一个亮点是应用了无痕保护基9-BBN(9-硼环[3.3.1]壬烷),











































 京公网安备 11010802027423号
京公网安备 11010802027423号