当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of N‐Fused 1,3‐Oxazolidines via Pd‐Catalyzed Decarboxylative (3+2) Cycloaddition
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901497 Jong‐Un Park 1 , Hye‐In Ahn 1 , Ho‐Jun Cho 1 , Zi Xuan 1 , Ju Hyun Kim 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-23 , DOI: 10.1002/adsc.201901497 Jong‐Un Park 1 , Hye‐In Ahn 1 , Ho‐Jun Cho 1 , Zi Xuan 1 , Ju Hyun Kim 1
Affiliation
|
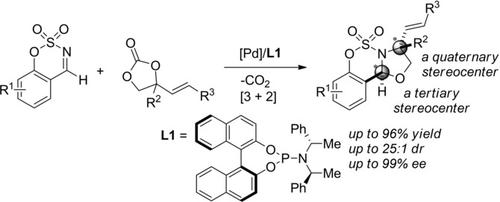
Efficient synthesis of optically active N‐fused 1,3‐oxazolidines containing quaternary and tertiary stereocenters was achieved via Pd‐catalyzed asymmetric (3+2) cycloadditions of sulfamate‐derived cyclic imines and vinylethylene carbonates. Using a chiral phosphoramidite ligand, the cycloadditions proceeded effectively providing sulfamidate‐fused 1,3‐oxazolidines in high yields (up to 96%) with stereoselectivities (up to 25:1 dr; >99% ee). Additionally, the scale‐up reaction and further transformations of the product were also achieved demonstrating the synthetic utility toward the construction of useful heterocycles such as chiral oxazoline bearing a quaternary stereocenter.
中文翻译:

通过Pd催化的脱羧(3 + 2)环加成反应不对称合成N-融合的1,3-恶唑烷
高效的合成方法是通过Pd催化氨基磺酸衍生的环状亚胺和碳酸乙烯基亚乙酯的Pd催化不对称(3 + 2)环加成反应,合成具有季和叔立体中心的旋光N-融合的1,3-恶唑烷。使用手性亚磷酰胺配体,可以有效地进行环加成反应,从而以高产率(高达96%)提供氨基磺酸酯融合的1,3-恶唑烷,并具有立体选择性(高达25:1 dr;> 99%ee)。此外,还实现了规模放大反应和产品的进一步转化,证明了其在构建有用的杂环(如带有四级立体中心的手性恶唑啉)方面的合成效用。
更新日期:2020-03-23
中文翻译:

通过Pd催化的脱羧(3 + 2)环加成反应不对称合成N-融合的1,3-恶唑烷
高效的合成方法是通过Pd催化氨基磺酸衍生的环状亚胺和碳酸乙烯基亚乙酯的Pd催化不对称(3 + 2)环加成反应,合成具有季和叔立体中心的旋光N-融合的1,3-恶唑烷。使用手性亚磷酰胺配体,可以有效地进行环加成反应,从而以高产率(高达96%)提供氨基磺酸酯融合的1,3-恶唑烷,并具有立体选择性(高达25:1 dr;> 99%ee)。此外,还实现了规模放大反应和产品的进一步转化,证明了其在构建有用的杂环(如带有四级立体中心的手性恶唑啉)方面的合成效用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号