当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PdII -Catalyzed Enantioselective C(sp3 )-H Arylation of Cyclobutyl Ketones Using a Chiral Transient Directing Group.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-10 , DOI: 10.1002/anie.202000532 Li-Jun Xiao 1 , Kai Hong 1 , Fan Luo 1 , Liang Hu 1 , William R Ewing 2 , Kap-Sun Yeung 3 , Jin-Quan Yu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-03-10 , DOI: 10.1002/anie.202000532 Li-Jun Xiao 1 , Kai Hong 1 , Fan Luo 1 , Liang Hu 1 , William R Ewing 2 , Kap-Sun Yeung 3 , Jin-Quan Yu 1
Affiliation
|
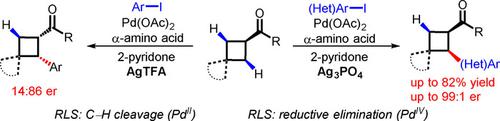
The use of chiral transient directing groups (TDGs) is a promising approach for developing PdII‐catalyzed enantioselective C(sp3)−H activation reactions. However, this strategy is challenging because the stereogenic center on the TDG is often far from the C−H bond, and both TDG covalently attached to the substrate and free TDG are capable of coordinating to PdII centers, which can result in a mixture of reactive complexes. We report a PdII‐catalyzed enantioselective β‐C(sp 3)−H arylation reaction of aliphatic ketones using a chiral TDG. A chiral trisubstituted cyclobutane was efficiently synthesized from a mono‐substituted cyclobutane through sequential C−H arylation reactions, thus demonstrating the utility of this method for accessing structurally complex products from simple starting materials. The use of an electron‐deficient pyridone ligand is crucial for the observed enantioselectivity. Interestingly, employing different silver salts can reverse the enantioselectivity.
中文翻译:

使用手性瞬态导向基团的 PdII 催化环丁基酮的对映选择性 C(sp3 )-H 芳基化。
使用手性瞬态导向基团 (TDG) 是开发 Pd II催化的对映选择性 C(sp 3 )−H 活化反应的一种有前途的方法。然而,这种策略具有挑战性,因为 TDG 上的立体中心通常远离 C−H 键,并且共价连接到底物上的 TDG 和游离 TDG 都能够与 Pd II中心配位,这可能导致混合反应复合物。我们报道了使用手性 TDG 的 Pd II催化的脂肪族酮的对映选择性 β-C( sp 3 )−H 芳基化反应。通过连续的 C-H 芳基化反应,由单取代环丁烷有效合成了手性三取代环丁烷,从而证明了该方法从简单起始原料获得结构复杂产物的实用性。缺电子吡啶酮配体的使用对于观察到的对映选择性至关重要。有趣的是,使用不同的银盐可以逆转对映选择性。
更新日期:2020-03-10
中文翻译:

使用手性瞬态导向基团的 PdII 催化环丁基酮的对映选择性 C(sp3 )-H 芳基化。
使用手性瞬态导向基团 (TDG) 是开发 Pd II催化的对映选择性 C(sp 3 )−H 活化反应的一种有前途的方法。然而,这种策略具有挑战性,因为 TDG 上的立体中心通常远离 C−H 键,并且共价连接到底物上的 TDG 和游离 TDG 都能够与 Pd II中心配位,这可能导致混合反应复合物。我们报道了使用手性 TDG 的 Pd II催化的脂肪族酮的对映选择性 β-C( sp 3 )−H 芳基化反应。通过连续的 C-H 芳基化反应,由单取代环丁烷有效合成了手性三取代环丁烷,从而证明了该方法从简单起始原料获得结构复杂产物的实用性。缺电子吡啶酮配体的使用对于观察到的对映选择性至关重要。有趣的是,使用不同的银盐可以逆转对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号