当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The anionic recognition mechanism based on polyol and boronic acid receptors
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/03/10 , DOI: 10.1039/c9nj06200a Renato Pereira Orenha 1, 2, 3, 4 , Claudia Haber Cintra 1, 2, 3, 4 , Letícia Bermudes Peixoto 1, 2, 3, 4 , Éder Henrique da Silva 1, 2, 3, 4 , Giovanni Finoto Caramori 4, 5, 6, 7 , Alexandre Osmar Ortolan 4, 5, 6, 7 , Maurício Jeomar Piotrowski 4, 8, 9, 10 , Renato Luis Tame Parreira 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/03/10 , DOI: 10.1039/c9nj06200a Renato Pereira Orenha 1, 2, 3, 4 , Claudia Haber Cintra 1, 2, 3, 4 , Letícia Bermudes Peixoto 1, 2, 3, 4 , Éder Henrique da Silva 1, 2, 3, 4 , Giovanni Finoto Caramori 4, 5, 6, 7 , Alexandre Osmar Ortolan 4, 5, 6, 7 , Maurício Jeomar Piotrowski 4, 8, 9, 10 , Renato Luis Tame Parreira 1, 2, 3, 4
Affiliation
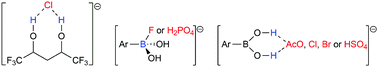
|
Anion selective recognition plays pivotal roles in the environmental field. The polyol and boronic acid receptor classes are investigated here to recognize the chloride (Cl−); fluoride (F−); dihydrogen phosphate (H2PO4−); acetate (AcO−); bromide (Br−); and hydrogen sulfate (HSO4−) anions. Energy Decomposition Analysis (EDA) shows that the anion–receptor interactions are predominantly electrostatic. Interestingly, the increase of the number of –CF3 groups or of the carbonic chain in the polyol structure favors Cl− recognition. The polyol–Cl− interactions are more attractive than the boronic acid–Cl− bond. Boronic acid recognizes F− and H2PO4− with the most attractive energies. The Quantum Theory of Atoms in Molecules (QTAIM) method elucidated that the recognition of AcO−, Cl−, Br−, and HSO4− by the receptors is maintained through hydrogen bonds, while the recognition of F− and H2PO4− by boronic acid involves B⋯F− and B⋯O−–P bonds, respectively, showing a larger covalent character compared to other boronic acid–anion bonds, but with a primarily electrostatic nature.
中文翻译:

基于多元醇和硼酸受体的阴离子识别机理
阴离子选择性识别在环境领域起着至关重要的作用。多元醇与硼酸受体类别在此研究以识别氯(氯-); 氟化物(F -); 磷酸二氢(H 2 PO 4 -); 乙酸酯(ACO -); 溴(溴-); 和硫酸氢(HSO 4 - )阴离子。能量分解分析(EDA)表明,阴离子与受体的相互作用主要为静电。有趣的是,-CF的数量的增加3基团或在多元醇结构中的碳链的有利于氯-认可。多元醇,氯-的相互作用比硼酸-CL更具吸引力-键。硼酸识别˚F -和H 2 PO 4 -与最有吸引力的能量。原子的分子的量子理论(QTAIM)方法阐明了识别ACO的- ,氯- ,溴-和HSO 4 -由所述受体是通过氢键保持,而F的识别-和H 2 PO 4 -由硼酸涉及乙⋯˚F -和B⋯ö -与其他硼酸-阴离子键相比,-P键分别显示出更大的共价特性,但主要具有静电性质。
更新日期:2020-04-06
中文翻译:

基于多元醇和硼酸受体的阴离子识别机理
阴离子选择性识别在环境领域起着至关重要的作用。多元醇与硼酸受体类别在此研究以识别氯(氯-); 氟化物(F -); 磷酸二氢(H 2 PO 4 -); 乙酸酯(ACO -); 溴(溴-); 和硫酸氢(HSO 4 - )阴离子。能量分解分析(EDA)表明,阴离子与受体的相互作用主要为静电。有趣的是,-CF的数量的增加3基团或在多元醇结构中的碳链的有利于氯-认可。多元醇,氯-的相互作用比硼酸-CL更具吸引力-键。硼酸识别˚F -和H 2 PO 4 -与最有吸引力的能量。原子的分子的量子理论(QTAIM)方法阐明了识别ACO的- ,氯- ,溴-和HSO 4 -由所述受体是通过氢键保持,而F的识别-和H 2 PO 4 -由硼酸涉及乙⋯˚F -和B⋯ö -与其他硼酸-阴离子键相比,-P键分别显示出更大的共价特性,但主要具有静电性质。









































 京公网安备 11010802027423号
京公网安备 11010802027423号