当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fused azole-thiazolines via one-pot cyclization of functionalized N-heterocyclic carbene precursors
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/10 , DOI: 10.1039/c9ob02548k Qiaoqiao Teng 1, 2, 3, 4 , Chandan Singh 1, 2, 3, 4 , Yuan Han 1, 2, 3, 4 , Han Vinh Huynh 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/10 , DOI: 10.1039/c9ob02548k Qiaoqiao Teng 1, 2, 3, 4 , Chandan Singh 1, 2, 3, 4 , Yuan Han 1, 2, 3, 4 , Han Vinh Huynh 1, 2, 3, 4
Affiliation
|
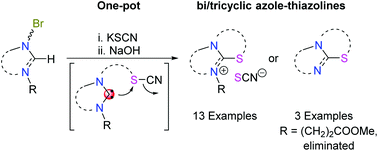
A one-pot two-step methodology was exploited to synthesize fused thiazoline-azolium salts via reactions of bromoalkyl-azolium salts with KSCN and NaOH. The synthetic feasibility and versatility was demonstrated by the high yield (>80%) preparation of 13 salts with different backbones, linkers and substituents. Using methylpropionato as an N-protecting group, the resulting salts could be further derivatized to their neutral azole-thiazolines. The reaction sequence proceeds via (i) Br → SCN substitution, (ii) N-heterocyclic carbene formation, (iii) carbene attack of the S atom and CN− displacement in the alkyl−S−C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N unit, and (iv) methyl acrylate elimination.
N unit, and (iv) methyl acrylate elimination.
中文翻译:

通过一锅环化功能化的N-杂环卡宾前体进行熔融的吡唑并噻唑啉
利用一锅两步法通过溴代烷基偶氮盐与KSCN和NaOH的反应合成稠合的噻唑啉-偶氮盐。高产率(> 80%)制备具有不同主链,连接基和取代基的13种盐证明了合成的可行性和多功能性。使用甲基丙酸酯作为N-保护基,可以将所得盐进一步衍生为它们的中性唑-噻唑啉。反应顺序进行通过(ⅰ)溴→SCN取代,(ⅱ)N-杂环卡宾的形成,(iii)所述S原子和CN的卡宾攻击-在烷基-S-C位移![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) N个单位,和(iv)甲基消除丙烯酸酯。
N个单位,和(iv)甲基消除丙烯酸酯。
更新日期:2020-04-01
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N unit, and (iv) methyl acrylate elimination.
N unit, and (iv) methyl acrylate elimination.
中文翻译:

通过一锅环化功能化的N-杂环卡宾前体进行熔融的吡唑并噻唑啉
利用一锅两步法通过溴代烷基偶氮盐与KSCN和NaOH的反应合成稠合的噻唑啉-偶氮盐。高产率(> 80%)制备具有不同主链,连接基和取代基的13种盐证明了合成的可行性和多功能性。使用甲基丙酸酯作为N-保护基,可以将所得盐进一步衍生为它们的中性唑-噻唑啉。反应顺序进行通过(ⅰ)溴→SCN取代,(ⅱ)N-杂环卡宾的形成,(iii)所述S原子和CN的卡宾攻击-在烷基-S-C位移
![[三重键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e002.gif) N个单位,和(iv)甲基消除丙烯酸酯。
N个单位,和(iv)甲基消除丙烯酸酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号