当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2-cis-Selective glucosylation enabled by halogenated benzyl protecting groups
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/10 , DOI: 10.1039/d0ob00373e Dancan K. Njeri 1, 2, 3, 4 , Claude J. Pertuit 1, 2, 3, 4 , Justin R. Ragains 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/10 , DOI: 10.1039/d0ob00373e Dancan K. Njeri 1, 2, 3, 4 , Claude J. Pertuit 1, 2, 3, 4 , Justin R. Ragains 1, 2, 3, 4
Affiliation
|
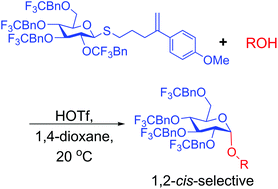
We report on our initial results from a systematic effort to implement electron-withdrawing protecting groups and Lewis basic solvents/additives as an approach to 1,2-cis(α)-selective O-glucosylation. 1,2-cis-Selective O-glucosylations are reported with thioglucosides and glucosyl trichloroacetimidates and a range of acceptors. A correlation between electron-withdrawing effects and 1,2-cis selectivity has been established. This phenomenon may prove to be broadly applicable in the area of chemical O-glycosylation.
中文翻译:

卤代苄基保护基团可实现1,2-顺式选择性糖基化
我们报告了我们的初步结果,这是系统地实施吸电子保护基团和Lewis碱性溶剂/添加剂作为1,2-顺式(α)-选择性O-葡萄糖基化方法的结果。据报道,硫代葡糖苷和葡糖基三氯乙酰亚氨酸酯和一系列受体具有1,2-顺式选择性O-葡糖基化。已经建立了吸电子效应与1,2-顺式选择性之间的相关性。该现象可能被证明可广泛用于化学O-糖基化领域。
更新日期:2020-04-01
中文翻译:

卤代苄基保护基团可实现1,2-顺式选择性糖基化
我们报告了我们的初步结果,这是系统地实施吸电子保护基团和Lewis碱性溶剂/添加剂作为1,2-顺式(α)-选择性O-葡萄糖基化方法的结果。据报道,硫代葡糖苷和葡糖基三氯乙酰亚氨酸酯和一系列受体具有1,2-顺式选择性O-葡糖基化。已经建立了吸电子效应与1,2-顺式选择性之间的相关性。该现象可能被证明可广泛用于化学O-糖基化领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号