Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual photothermal MDSCs-targeted immunotherapy inhibits lung immunosuppressive metastasis by enhancing T-cell recruitment
Nanoscale ( IF 5.8 ) Pub Date : 2020/03/10 , DOI: 10.1039/d0nr00080a Kalliopi Domvri 1, 2, 3, 4, 5 , Savvas Petanidis 3, 4, 5, 6, 7 , Doxakis Anestakis 3, 4, 5, 6, 7 , Konstantinos Porpodis 1, 2, 3, 4, 5 , Chong Bai 8, 9, 10, 11, 12 , Paul Zarogoulidis 3, 5, 13, 14, 15 , Lutz Freitag 16, 17, 18, 19 , Wolfgang Hohenforst-Schmidt 20, 21, 22, 23, 24 , Theodora Katopodi 3, 4, 5, 6, 7
Nanoscale ( IF 5.8 ) Pub Date : 2020/03/10 , DOI: 10.1039/d0nr00080a Kalliopi Domvri 1, 2, 3, 4, 5 , Savvas Petanidis 3, 4, 5, 6, 7 , Doxakis Anestakis 3, 4, 5, 6, 7 , Konstantinos Porpodis 1, 2, 3, 4, 5 , Chong Bai 8, 9, 10, 11, 12 , Paul Zarogoulidis 3, 5, 13, 14, 15 , Lutz Freitag 16, 17, 18, 19 , Wolfgang Hohenforst-Schmidt 20, 21, 22, 23, 24 , Theodora Katopodi 3, 4, 5, 6, 7
Affiliation
|
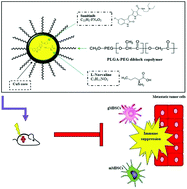
Immunosuppressive chemoresistance is a major barrier in lung cancer treatment. However, the immunosuppressive mechanisms responsible for lung cancer cell chemoresistance and tumor relapse are still unknown. In this study, we introduce a model of precise immunosuppressive-based nanotherapy by designing and delivering biocompatible MDSC-targeted nanocarriers (NCs) into the lung tumor microenvironment. This is accomplished by conjugating L-Norvaline and Sunitinib integrated into biodegradable nanosomes in order to facilitate inhibition of tumor-supporting immunosuppression. Findings show that treatment with NCs increased apoptosis and significantly reduced tumor volume and Ki-67 antigen expression respectively. Biodistribution analysis revealed an increase in drug circulation time, as well as a greater accumulation in lung and peripheral tissues. Furthermore, an upregulation of tumor infiltrating lymphocytes expression was observed, especially CD8+ T cells by 27%, and CD4+ T cells by 7% compared to PBS treatment. The presence of CD161+ (NK1.1) cells revealed NK cell activation followed by decreased MDSC infiltration and MDSC subsets were characterized by the reduction of Gr/CD11b cell population in blood and tissue samples. In addition, these nanospheres, showed increased PTT efficiency and tumour targeting ability as evidenced by highly efficient tumour ablation under near infrared (NIR) exposure. Significant tumor reduction was observed due to recruitment of cytotoxic T-lymphocytes, followed by downregulation of immunosuppressive Foxp3+ Treg cells. Taken together, our findings provide a novel nanodrug delivery strategy for the inhibition of MDSC-related immunosuppression in lung tumor microenvironment and provide a new approach for the efficient treatment of metastatic cancer.
中文翻译:

靶向双光热MDSC的免疫疗法可通过增强T细胞募集来抑制肺部免疫抑制转移
免疫抑制化学耐药性是肺癌治疗的主要障碍。然而,导致肺癌细胞化学抗性和肿瘤复发的免疫抑制机制仍是未知的。在这项研究中,我们通过设计生物相容性MDSC靶向纳米载体(NCs)并将其传递到肺肿瘤微环境中,引入了一种基于精确免疫抑制的纳米疗法模型。这是通过共轭L来完成的-去甲缬氨酸和舒尼替尼被整合到可生物降解的纳米体内,以促进抑制肿瘤支持的免疫抑制。研究结果表明,NCs治疗可增加细胞凋亡,并显着降低肿瘤体积和Ki-67抗原表达。生物分布分析表明,药物循环时间增加,在肺和周围组织中的累积也更多。此外,与PBS治疗相比,观察到肿瘤浸润淋巴细胞表达的上调,特别是CD8 + T细胞增加了27%,CD4 + T细胞增加了7%。CD161 +的存在(NK1.1)细胞显示NK细胞活化后,MDSC浸润减少,MDSC亚集的特征是血液和组织样品中Gr / CD11b细胞的减少。此外,这些纳米球表现出提高的PTT效率和靶向肿瘤的能力,这在近红外(NIR)照射下高效消融肿瘤得以证明。由于募集了细胞毒性T淋巴细胞,随后免疫抑制性Foxp3 + Treg细胞下调,观察到明显的肿瘤减少。综上所述,我们的发现为抑制肺癌微环境中MDSC相关的免疫抑制提供了一种新颖的纳米药物递送策略,并为有效治疗转移性癌症提供了一种新方法。
更新日期:2020-04-03
中文翻译:

靶向双光热MDSC的免疫疗法可通过增强T细胞募集来抑制肺部免疫抑制转移
免疫抑制化学耐药性是肺癌治疗的主要障碍。然而,导致肺癌细胞化学抗性和肿瘤复发的免疫抑制机制仍是未知的。在这项研究中,我们通过设计生物相容性MDSC靶向纳米载体(NCs)并将其传递到肺肿瘤微环境中,引入了一种基于精确免疫抑制的纳米疗法模型。这是通过共轭L来完成的-去甲缬氨酸和舒尼替尼被整合到可生物降解的纳米体内,以促进抑制肿瘤支持的免疫抑制。研究结果表明,NCs治疗可增加细胞凋亡,并显着降低肿瘤体积和Ki-67抗原表达。生物分布分析表明,药物循环时间增加,在肺和周围组织中的累积也更多。此外,与PBS治疗相比,观察到肿瘤浸润淋巴细胞表达的上调,特别是CD8 + T细胞增加了27%,CD4 + T细胞增加了7%。CD161 +的存在(NK1.1)细胞显示NK细胞活化后,MDSC浸润减少,MDSC亚集的特征是血液和组织样品中Gr / CD11b细胞的减少。此外,这些纳米球表现出提高的PTT效率和靶向肿瘤的能力,这在近红外(NIR)照射下高效消融肿瘤得以证明。由于募集了细胞毒性T淋巴细胞,随后免疫抑制性Foxp3 + Treg细胞下调,观察到明显的肿瘤减少。综上所述,我们的发现为抑制肺癌微环境中MDSC相关的免疫抑制提供了一种新颖的纳米药物递送策略,并为有效治疗转移性癌症提供了一种新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号