当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the effect of N(ε)-(carboxyethyl)lysine on the conformation, dynamics and aggregation propensity of α-synuclein
Chemical Science ( IF 7.6 ) Pub Date : 2020/03/10 , DOI: 10.1039/d0sc00906g Laura Mariño 1, 2, 3, 4, 5 , Rafael Ramis 1, 2, 3, 4, 5 , Rodrigo Casasnovas 1, 2, 3, 4, 5 , Joaquín Ortega-Castro 1, 2, 3, 4, 5 , Bartolomé Vilanova 1, 2, 3, 4, 5 , Juan Frau 1, 2, 3, 4, 5 , Miquel Adrover 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2020/03/10 , DOI: 10.1039/d0sc00906g Laura Mariño 1, 2, 3, 4, 5 , Rafael Ramis 1, 2, 3, 4, 5 , Rodrigo Casasnovas 1, 2, 3, 4, 5 , Joaquín Ortega-Castro 1, 2, 3, 4, 5 , Bartolomé Vilanova 1, 2, 3, 4, 5 , Juan Frau 1, 2, 3, 4, 5 , Miquel Adrover 1, 2, 3, 4, 5
Affiliation
|
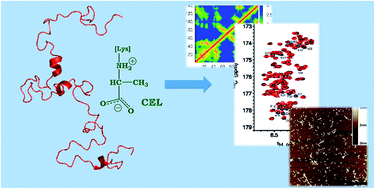
α-Synuclein (αS) aggregation is a hallmark in several neurodegenerative diseases. Among them, Parkinson's disease is highlighted, characterized by the intraneuronal deposition of Lewy bodies (LBs) which causes the loss of dopaminergic neurons. αS is the main component of LBs and in them, it usually contains post-translational modifications. One of them is the formation of advanced glycation end-products (mainly CEL and MOLD) arising from its reaction with methylglyoxal. Despite its biological relevance, there are no data available proving the effect of glycation on the conformation of αS, nor on its aggregation mechanism. This has been hampered by the formation of a heterogeneous set of compounds that precluded conformational studies. To overcome this issue, we have here produced αS homogeneously glycated with CEL. Its use, together with different biophysical techniques and molecular dynamics simulations, allowed us to study for the first time the effect of glycation on the conformation of a protein. CEL extended the conformation of the N-terminal domain as a result of the loss of transient N-/C-terminal long-range contacts while increasing the heterogeneity of the conformational population. CEL also inhibited the αS aggregation, but it was not able to disassemble preexisting amyloid fibrils, thus proving that CEL found on LBs must be formed in a later event after aggregation.
中文翻译:

阐明N(ε)-(羧乙基)赖氨酸对α-突触核蛋白的构象,动力学和聚集倾向的影响
α-突触核蛋白(αS)聚集是几种神经退行性疾病的标志。其中,帕金森氏病是突出的,其特征是路易体(LB)的神经内沉积会导致多巴胺能神经元的丢失。αS是LB的主要成分,并且在其中通常包含翻译后修饰。其中之一是由于其与甲基乙二醛反应而形成的高级糖基化终产物(主要是CEL和MOLD)。尽管它具有生物学相关性,但尚无可用数据证明糖基化对αS构象及其聚集机理的影响。这已经因无法进行构象研究的异质化合物的形成而受到阻碍。为了克服这个问题,我们在这里生产了被CEL均匀糖化的αS。它的用途 结合不同的生物物理技术和分子动力学模拟,使我们首次研究了糖基化对蛋白质构象的影响。CEL扩展了N末端域的构象,这是由于失去了瞬时N- / C末端远程接触而导致的,同时增加了构象种群的异质性。CEL还抑制了αS聚集,但是它不能分解先前存在的淀粉样原纤维,因此证明了在LBs上发现的CEL必须在聚集后的以后事件中形成。由于瞬时N- / C末端远程接触的丧失,CEL扩展了N末端结构域的构象,同时增加了构象种群的异质性。CEL还抑制了αS聚集,但是它无法分解先前存在的淀粉样原纤维,因此证明了在LBs上发现的CEL必须在聚集后的以后事件中形成。CEL扩展了N末端域的构象,这是由于失去了瞬时N- / C末端远程接触而导致的,同时增加了构象种群的异质性。CEL还抑制了αS聚集,但是它无法分解先前存在的淀粉样原纤维,因此证明了在LBs上发现的CEL必须在聚集后的以后事件中形成。
更新日期:2020-03-26
中文翻译:

阐明N(ε)-(羧乙基)赖氨酸对α-突触核蛋白的构象,动力学和聚集倾向的影响
α-突触核蛋白(αS)聚集是几种神经退行性疾病的标志。其中,帕金森氏病是突出的,其特征是路易体(LB)的神经内沉积会导致多巴胺能神经元的丢失。αS是LB的主要成分,并且在其中通常包含翻译后修饰。其中之一是由于其与甲基乙二醛反应而形成的高级糖基化终产物(主要是CEL和MOLD)。尽管它具有生物学相关性,但尚无可用数据证明糖基化对αS构象及其聚集机理的影响。这已经因无法进行构象研究的异质化合物的形成而受到阻碍。为了克服这个问题,我们在这里生产了被CEL均匀糖化的αS。它的用途 结合不同的生物物理技术和分子动力学模拟,使我们首次研究了糖基化对蛋白质构象的影响。CEL扩展了N末端域的构象,这是由于失去了瞬时N- / C末端远程接触而导致的,同时增加了构象种群的异质性。CEL还抑制了αS聚集,但是它不能分解先前存在的淀粉样原纤维,因此证明了在LBs上发现的CEL必须在聚集后的以后事件中形成。由于瞬时N- / C末端远程接触的丧失,CEL扩展了N末端结构域的构象,同时增加了构象种群的异质性。CEL还抑制了αS聚集,但是它无法分解先前存在的淀粉样原纤维,因此证明了在LBs上发现的CEL必须在聚集后的以后事件中形成。CEL扩展了N末端域的构象,这是由于失去了瞬时N- / C末端远程接触而导致的,同时增加了构象种群的异质性。CEL还抑制了αS聚集,但是它无法分解先前存在的淀粉样原纤维,因此证明了在LBs上发现的CEL必须在聚集后的以后事件中形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号