PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-09 , DOI: 10.1371/journal.ppat.1008373 David Oyen 1 , Jonathan L Torres 1 , Phillip C Aoto 2 , Yevel Flores-Garcia 3 , Špela Binter 4 , Tossapol Pholcharee 1 , Sean Carroll 5 , Sini Reponen 5 , Rachael Wash 4 , Qi Liang 4 , Franck Lemiale 6 , Emily Locke 6 , Allan Bradley 4, 7 , C Richter King 6 , Daniel Emerling 5 , Paul Kellam 4, 8 , Fidel Zavala 3 , Andrew B Ward 1 , Ian A Wilson 1, 9
|
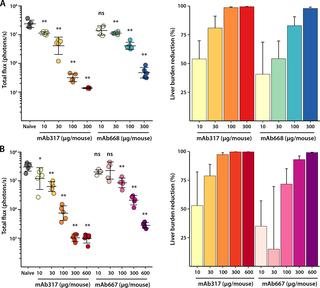
Lasting protection has long been a goal for malaria vaccines. The major surface antigen on Plasmodium falciparum sporozoites, the circumsporozoite protein (PfCSP), has been an attractive target for vaccine development and most protective antibodies studied to date interact with the central NANP repeat region of PfCSP. However, it remains unclear what structural and functional characteristics correlate with better protection by one antibody over another. Binding to the junctional region between the N-terminal domain and central NANP repeats has been proposed to result in superior protection: this region initiates with the only NPDP sequence followed immediately by NANP. Here, we isolated antibodies in Kymab mice immunized with full-length recombinant PfCSP and two protective antibodies were selected for further study with reactivity against the junctional region. X-ray and EM structures of two monoclonal antibodies, mAb667 and mAb668, shed light on their differential affinity and specificity for the junctional region. Importantly, these antibodies also bind to the NANP repeat region with equal or better affinity. A comparison with an NANP-only binding antibody (mAb317) revealed roughly similar but statistically distinct levels of protection against sporozoite challenge in mouse liver burden models, suggesting that junctional antibody protection might relate to the ability to also cross-react with the NANP repeat region. Our findings indicate that additional efforts are necessary to isolate a true junctional antibody with no or much reduced affinity to the NANP region to elucidate the role of the junctional epitope in protection.
中文翻译:

单克隆抗体与恶性疟原虫环子孢子蛋白结合表位结合的结构和机理。
长期保护长期以来一直是疟疾疫苗的目标。恶性疟原虫上的主要表面抗原子孢子,环子孢子蛋白(PfCSP),已成为疫苗开发的有吸引力的靶标,迄今为止研究的大多数保护性抗体都与PfCSP的中央NANP重复区域相互作用。然而,尚不清楚哪种结构和功能特征与一种抗体相对于另一种抗体的更好保护相关。已经提出结合至N-末端结构域和中央NANP重复序列之间的接合区域以产生更好的保护:该区域以唯一的NPDP序列开始,紧接着是NANP。在这里,我们在用全长重组PfCSP免疫的Kymab小鼠中分离了抗体,并选择了两种保护性抗体用于进一步研究,其对连接区具有反应性。两种单克隆抗体mAb667和mAb668的X射线和EM结构 揭示了它们对连接区的不同亲和力和特异性。重要的是,这些抗体还以相等或更好的亲和力结合到NANP重复区域。与仅NANP的结合抗体(mAb317)的比较显示,在小鼠肝脏负荷模型中,对子孢子攻击的保护水平大致相似,但在统计学上不同,这表明连接抗体的保护可能与与NANP重复区域交叉反应的能力有关。 。我们的发现表明,需要做出额外的努力来分离对NANP区没有亲和力或亲和力降低的真正的连接抗体,以阐明连接表位在保护中的作用。这些抗体也以相等或更好的亲和力结合到NANP重复区域。与仅NANP的结合抗体(mAb317)的比较显示,在小鼠肝脏负荷模型中,对子孢子攻击的保护水平大致相似,但在统计学上不同,这表明连接抗体的保护可能与与NANP重复区域交叉反应的能力有关。 。我们的发现表明,需要做出额外的努力来分离对NANP区没有亲和力或亲和力降低的真正的连接抗体,以阐明连接表位在保护中的作用。这些抗体也以相等或更好的亲和力结合到NANP重复区域。与仅NANP的结合抗体(mAb317)的比较显示,在小鼠肝脏负荷模型中,对子孢子攻击的保护水平大致相似,但在统计学上不同,这表明连接抗体的保护可能与与NANP重复区域交叉反应的能力有关。 。我们的发现表明,需要做出额外的努力来分离对NANP区没有亲和力或亲和力降低的真正的连接抗体,以阐明连接表位在保护中的作用。提示连接抗体保护可能与NANP重复区域也发生交叉反应的能力有关。我们的发现表明,需要做出额外的努力来分离对NANP区域无亲和力或亲和力降低的真正的连接抗体,以阐明连接表位在保护中的作用。提示连接抗体保护可能与NANP重复区域也发生交叉反应的能力有关。我们的发现表明,需要做出额外的努力来分离对NANP区没有亲和力或亲和力降低的真正的连接抗体,以阐明连接表位在保护中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号