Mucosal Immunology ( IF 7.9 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41385-020-0279-5 Leila B Giron 1 , Ceylan E Tanes 2 , Mariane H Schleimann 3 , Phillip A Engen 4 , Lisa M Mattei 2 , Alitzel Anzurez 1 , Mohammad Damra 1 , Huanjia Zhang 2 , Kyle Bittinger 2 , Frederic Bushman 5 , Andrew Kossenkov 1 , Paul W Denton 6 , Hiroaki Tateno 7 , Ali Keshavarzian 4 , Alan L Landay 8 , Mohamed Abdel-Mohsen 1
|
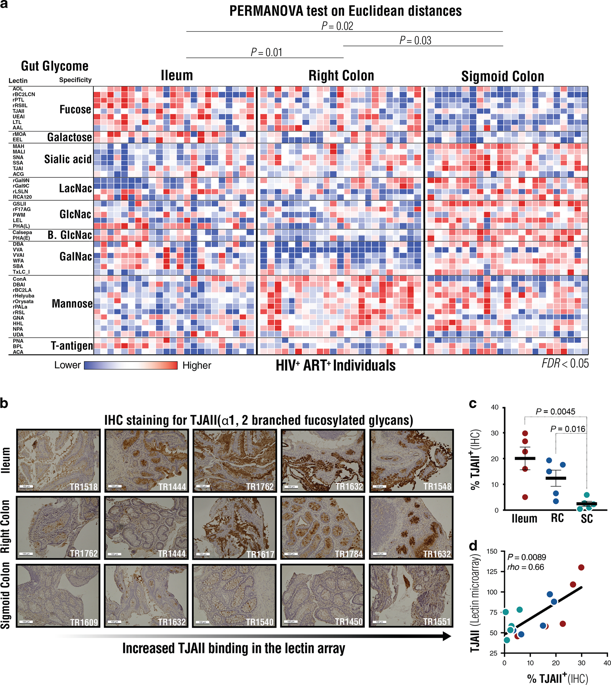
An emerging paradigm suggests that gut glycosylation is a key force in maintaining the homeostatic relationship between the gut and its microbiota. Nevertheless, it is unclear how gut glycosylation contributes to the HIV-associated microbial translocation and inflammation that persist despite viral suppression and contribute to the development of several comorbidities. We examined terminal ileum, right colon, and sigmoid colon biopsies from HIV-infected virally-suppressed individuals and found that gut glycomic patterns are associated with distinct microbial compositions and differential levels of chronic inflammation and HIV persistence. In particular, high levels of the pro-inflammatory hypo-sialylated T-antigen glycans and low levels of the anti-inflammatory fucosylated glycans were associated with higher abundance of glycan-degrading microbial species (in particular, Bacteroides vulgatus), a less diverse microbiome, higher levels of inflammation, and higher levels of ileum-associated HIV DNA. These findings are linked to the activation of the inflammasome-mediating eIF2 signaling pathway. Our study thus provides the first proof-of-concept evidence that a previously unappreciated factor, gut glycosylation, is a force that may impact the vicious cycle between HIV infection, microbial translocation, and chronic inflammation.
中文翻译:

唾液酸化和岩藻糖基化在 HIV 感染期间调节炎性体激活 eIF2 信号和微生物易位。
一种新兴的范例表明,肠道糖基化是维持肠道与其微生物群之间稳态关系的关键力量。然而,目前尚不清楚肠道糖基化如何导致 HIV 相关微生物易位和炎症,尽管病毒受到抑制,但炎症仍然存在,并导致多种合并症的发生。我们检查了来自 HIV 感染的病毒抑制个体的末端回肠、右结肠和乙状结肠活检,发现肠道糖组学模式与不同的微生物组成和不同水平的慢性炎症和 HIV 持续存在有关。尤其,Bacteroides vulgatus),微生物组多样性较低,炎症水平较高,回肠相关 HIV DNA 水平较高。这些发现与炎症小体介导的 eIF2 信号通路的激活有关。因此,我们的研究提供了第一个概念验证证据,证明以前未被重视的因素肠道糖基化是一种可能影响 HIV 感染、微生物易位和慢性炎症之间恶性循环的力量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号