Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ADAR1-mediated RNA editing is a novel oncogenic process in thyroid cancer and regulates miR-200 activity.
Oncogene ( IF 6.9 ) Pub Date : 2020-03-10 , DOI: 10.1038/s41388-020-1248-x Julia Ramírez-Moya 1, 2, 3 , Allison R Baker 2 , Frank J Slack 2 , Pilar Santisteban 1, 3
Oncogene ( IF 6.9 ) Pub Date : 2020-03-10 , DOI: 10.1038/s41388-020-1248-x Julia Ramírez-Moya 1, 2, 3 , Allison R Baker 2 , Frank J Slack 2 , Pilar Santisteban 1, 3
Affiliation
|
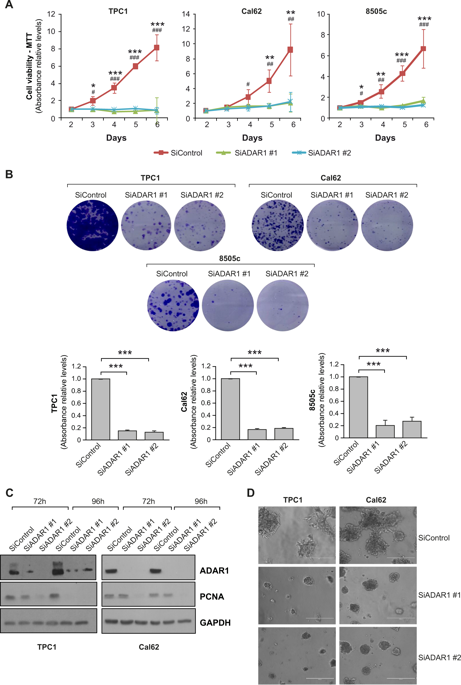
Adenosine deaminases acting on RNA (ADARs) convert adenosine to inosine in double-stranded RNA. A-to-I editing of RNA is a widespread posttranscriptional process that has recently emerged as an important mechanism in cancer biology. A-to-I editing levels are high in several human cancers, including thyroid cancer, but ADAR1 editase-dependent mechanisms governing thyroid cancer progression are unexplored. To address the importance of RNA A-to-I editing in thyroid cancer, we examined the role of ADAR1. Loss-of-function analysis showed that ADAR1 suppression profoundly repressed proliferation, invasion, and migration in thyroid tumor cell models. These observations were validated in an in vivo xenograft model, which showed that ADAR1-silenced cells had a diminished ability to form tumors. RNA editing of miRNAs has the potential to markedly alter target recognition. According to TCGA data, the tumor suppressor miR-200b is overedited in thyroid tumors, and its levels of editing correlate with a worse progression-free survival and disease stage. We confirmed miR-200b overediting in thyroid tumors and we showed that edited miR-200b has weakened activity against its target gene ZEB1 in thyroid cancer cells, likely explaining the reduced aggressiveness of ADAR1-silenced cells. We also found that RAS, but not BRAF, modulates ADAR1 levels, an effect mediated predominantly by PI3K and in part by MAPK. Lastly, pharmacological inhibition of ADAR1 activity with the editing inhibitor 8-azaadenosine reduced cancer cell aggressiveness. Overall, our data implicate ADAR1-mediated A-to-I editing as an important pathway in thyroid cancer progression, and highlight RNA editing as a potential therapeutic target in thyroid cancer.
中文翻译:

ADAR1 介导的 RNA 编辑是甲状腺癌中一种新型致癌过程,可调节 miR-200 活性。
作用于 RNA 的腺苷脱氨酶 (ADAR) 将双链 RNA 中的腺苷转化为肌苷。RNA 的 A 到 I 编辑是一种广泛存在的转录后过程,最近已成为癌症生物学中的重要机制。A-to-I 编辑水平在包括甲状腺癌在内的多种人类癌症中都很高,但控制甲状腺癌进展的 ADAR1 编辑酶依赖性机制尚未被探索。为了阐明 RNA A 至 I 编辑在甲状腺癌中的重要性,我们研究了 ADAR1 的作用。功能丧失分析表明,ADAR1 抑制可深度抑制甲状腺肿瘤细胞模型的增殖、侵袭和迁移。这些观察结果在体内异种移植模型中得到了验证,该模型表明 ADAR1 沉默的细胞形成肿瘤的能力减弱。miRNA 的 RNA 编辑有可能显着改变目标识别。根据 TCGA 数据,肿瘤抑制因子 miR-200b 在甲状腺肿瘤中被过度编辑,其编辑水平与较差的无进展生存期和疾病阶段相关。我们证实了甲状腺肿瘤中的 miR-200b 过度编辑,并且我们发现编辑后的 miR-200b 削弱了甲状腺癌细胞中针对其靶基因 ZEB1 的活性,这可能解释了 ADAR1 沉默细胞的攻击性降低。我们还发现 RAS(而非 BRAF)调节 ADAR1 水平,这种效应主要由 PI3K 介导,部分由 MAPK 介导。最后,使用编辑抑制剂 8-氮杂腺苷对 ADAR1 活性进行药理抑制可降低癌细胞的侵袭性。总体而言,我们的数据表明 ADAR1 介导的 A 至 I 编辑是甲状腺癌进展的重要途径,
更新日期:2020-03-10
中文翻译:

ADAR1 介导的 RNA 编辑是甲状腺癌中一种新型致癌过程,可调节 miR-200 活性。
作用于 RNA 的腺苷脱氨酶 (ADAR) 将双链 RNA 中的腺苷转化为肌苷。RNA 的 A 到 I 编辑是一种广泛存在的转录后过程,最近已成为癌症生物学中的重要机制。A-to-I 编辑水平在包括甲状腺癌在内的多种人类癌症中都很高,但控制甲状腺癌进展的 ADAR1 编辑酶依赖性机制尚未被探索。为了阐明 RNA A 至 I 编辑在甲状腺癌中的重要性,我们研究了 ADAR1 的作用。功能丧失分析表明,ADAR1 抑制可深度抑制甲状腺肿瘤细胞模型的增殖、侵袭和迁移。这些观察结果在体内异种移植模型中得到了验证,该模型表明 ADAR1 沉默的细胞形成肿瘤的能力减弱。miRNA 的 RNA 编辑有可能显着改变目标识别。根据 TCGA 数据,肿瘤抑制因子 miR-200b 在甲状腺肿瘤中被过度编辑,其编辑水平与较差的无进展生存期和疾病阶段相关。我们证实了甲状腺肿瘤中的 miR-200b 过度编辑,并且我们发现编辑后的 miR-200b 削弱了甲状腺癌细胞中针对其靶基因 ZEB1 的活性,这可能解释了 ADAR1 沉默细胞的攻击性降低。我们还发现 RAS(而非 BRAF)调节 ADAR1 水平,这种效应主要由 PI3K 介导,部分由 MAPK 介导。最后,使用编辑抑制剂 8-氮杂腺苷对 ADAR1 活性进行药理抑制可降低癌细胞的侵袭性。总体而言,我们的数据表明 ADAR1 介导的 A 至 I 编辑是甲状腺癌进展的重要途径,











































 京公网安备 11010802027423号
京公网安备 11010802027423号