当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deep mutational scanning reveals the structural basis for α-synuclein activity.
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41589-020-0480-6 Robert W Newberry 1 , Jaime T Leong 2 , Eric D Chow 3 , Martin Kampmann 2, 3, 4 , William F DeGrado 1
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41589-020-0480-6 Robert W Newberry 1 , Jaime T Leong 2 , Eric D Chow 3 , Martin Kampmann 2, 3, 4 , William F DeGrado 1
Affiliation
|
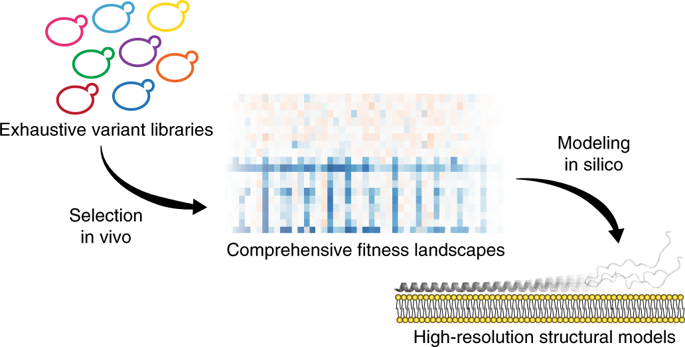
Defining the biologically active structures of proteins in their cellular environments remains challenging for proteins with multiple conformations and functions, where only a minor conformer might be associated with a given function. Here, we use deep mutational scanning to probe the structure and dynamics of α-synuclein, a protein known to adopt disordered, helical and amyloid conformations. We examined the effects of 2,600 single-residue substitutions on the ability of intracellularly expressed α-synuclein to slow the growth of yeast. Computational analysis of the data showed that the conformation responsible for this phenotype is a long, uninterrupted, amphiphilic helix with increasing dynamics toward the C terminus. Deep mutational scanning can therefore determine biologically active conformations in cellular environments, even for a highly dynamic multi-conformational protein.
中文翻译:

深度突变扫描揭示了 α-突触核蛋白活性的结构基础。
对于具有多种构象和功能的蛋白质来说,在细胞环境中定义蛋白质的生物活性结构仍然具有挑战性,其中只有较小的构象异构体可能与给定的功能相关。在这里,我们使用深度突变扫描来探测 α-突触核蛋白的结构和动力学,α-突触核蛋白是一种已知采用无序、螺旋和淀粉样蛋白构象的蛋白质。我们研究了 2,600 个单残基取代对细胞内表达的 α-突触核蛋白减缓酵母生长的能力的影响。数据的计算分析表明,造成这种表型的构象是一个长的、不间断的两亲性螺旋,其向 C 末端的动态增加。因此,深度突变扫描可以确定细胞环境中的生物活性构象,甚至对于高度动态的多构象蛋白质也是如此。
更新日期:2020-04-24
中文翻译:

深度突变扫描揭示了 α-突触核蛋白活性的结构基础。
对于具有多种构象和功能的蛋白质来说,在细胞环境中定义蛋白质的生物活性结构仍然具有挑战性,其中只有较小的构象异构体可能与给定的功能相关。在这里,我们使用深度突变扫描来探测 α-突触核蛋白的结构和动力学,α-突触核蛋白是一种已知采用无序、螺旋和淀粉样蛋白构象的蛋白质。我们研究了 2,600 个单残基取代对细胞内表达的 α-突触核蛋白减缓酵母生长的能力的影响。数据的计算分析表明,造成这种表型的构象是一个长的、不间断的两亲性螺旋,其向 C 末端的动态增加。因此,深度突变扫描可以确定细胞环境中的生物活性构象,甚至对于高度动态的多构象蛋白质也是如此。











































 京公网安备 11010802027423号
京公网安备 11010802027423号