The Lancet Oncology ( IF 41.6 ) Pub Date : 2020-03-09 , DOI: 10.1016/s1470-2045(19)30862-9 Chiara Cremolini 1 , Carlotta Antoniotti 1 , Daniele Rossini 1 , Sara Lonardi 2 , Fotios Loupakis 2 , Filippo Pietrantonio 3 , Roberto Bordonaro 4 , Tiziana Pia Latiano 5 , Emiliano Tamburini 6 , Daniele Santini 7 , Alessandro Passardi 8 , Federica Marmorino 1 , Roberta Grande 9 , Giuseppe Aprile 10 , Alberto Zaniboni 11 , Sabina Murgioni 2 , Cristina Granetto 12 , Angela Buonadonna 13 , Roberto Moretto 14 , Salvatore Corallo 15 , Stefano Cordio 4 , Lorenzo Antonuzzo 16 , Gianluca Tomasello 17 , Gianluca Masi 1 , Monica Ronzoni 18 , Samantha Di Donato 19 , Chiara Carlomagno 20 , Matteo Clavarezza 21 , Giuliana Ritorto 22 , Andrea Mambrini 23 , Mario Roselli 24 , Samanta Cupini 25 , Serafina Mammoliti 26 , Elisabetta Fenocchio 27 , Enrichetta Corgna 28 , Vittorina Zagonel 2 , Gabriella Fontanini 29 , Clara Ugolini 30 , Luca Boni 31 , Alfredo Falcone 1 ,
|
Background
The triplet FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) plus bevacizumab showed improved outcomes for patients with metastatic colorectal cancer, compared with FOLFIRI (fluorouracil, leucovorin, and irinotecan) plus bevacizumab. However, the actual benefit of the upfront exposure to the three cytotoxic drugs compared with a preplanned sequential strategy of doublets was not clear, and neither was the feasibility or efficacy of therapies after disease progression. We aimed to compare a preplanned strategy of upfront FOLFOXIRI followed by the reintroduction of the same regimen after disease progression versus a sequence of mFOLFOX6 (fluorouracil, leucovorin, and oxaliplatin) and FOLFIRI doublets, in combination with bevacizumab.
Methods
TRIBE2 was an open-label, phase 3, randomised study of patients aged 18–75 years with an Eastern Cooperative Oncology Group (ECOG) performance status of 2, with unresectable, previously untreated metastatic colorectal cancer, recruited from 58 Italian oncology units. Patients were stratified according to centre, ECOG performance status, primary tumour location, and previous adjuvant chemotherapy. A randomisation system incorporating a minimisation algorithm was used to randomly assign patients (1:1) via a masked web-based allocation procedure to two different treatment strategies. In the control group, patients received first-line mFOLFOX6 (85 mg/m2 of intravenous oxaliplatin concurrently with 200 mg/m2 of leucovorin over 120 min; 400 mg/m2 intravenous bolus of fluorouracil; 2400 mg/m2 continuous infusion of fluorouracil for 48 h) plus bevacizumab (5 mg/kg intravenously over 30 min) followed by FOLFIRI (180 mg/m2 of intravenous irinotecan over 120 min concurrently with 200 mg/m2 of leucovorin; 400 mg/m2 intravenous bolus of fluorouracil; 2400 mg/m2 continuous infusion of fluorouracil for 48 h) plus bevacizumab after disease progression. In the experimental group, patients received FOLFOXIRI (165 mg/m2 of intravenous irinotecan over 60 min; 85 mg/m2 intravenous oxaliplatin concurrently with 200 mg/m2 of leucovorin over 120 min; 3200 mg/m2 continuous infusion of fluorouracil for 48 h) plus bevacizumab followed by the reintroduction of the same regimen after disease progression. Combination treatments were repeated every 14 days for up to eight cycles followed by fluorouracil and leucovorin (at the same dose administered at the last induction cycle) plus bevacizumab maintenance until disease progression, unacceptable adverse events, or consent withdrawal. Patients and investigators were not masked. The primary endpoint was progression-free survival 2, defined as the time from randomisation to disease progression on any treatment given after first disease progression, or death, analysed by intention to treat. Safety was assessed in patients who received at least one dose of their assigned treatment. Study recruitment is complete and follow-up is ongoing. This trial is registered with Clinicaltrials.gov, NCT02339116.
Findings
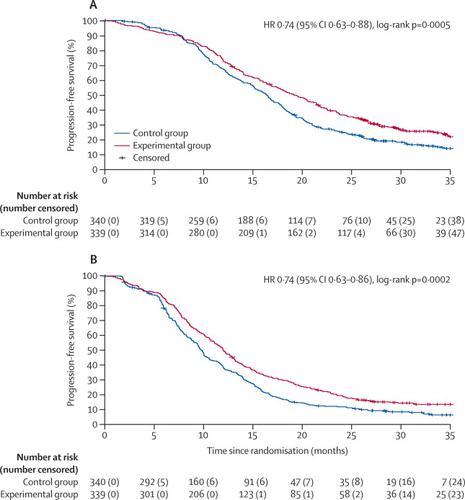
Between Feb 26, 2015, and May 15, 2017, 679 patients were randomly assigned and received treatment (340 in the control group and 339 in the experimental group). At data cut-off (July 30, 2019) median follow-up was 35·9 months (IQR 30·1–41·4). Median progression-free survival 2 was 19·2 months (95% CI 17·3–21·4) in the experimental group and 16·4 months (15·1–17·5) in the control group (hazard ratio [HR] 0·74, 95% CI 0·63–0·88; p=0·0005). During the first-line treatment, the most frequent of all-cause grade 3–4 events were diarrhoea (57 [17%] vs 18 [5%]), neutropenia (168 [50%] vs 71 [21%]), and arterial hypertension (25 [7%] vs 35 [10%]) in the experimental group compared with the control group. Serious adverse events occurred in 84 (25%) patients in the experimental group and in 56 (17%) patients in the control group. Eight treatment-related deaths were reported in the experimental group (two intestinal occlusions, two intestinal perforations, two sepsis, one myocardial infarction, and one bleeding) and four in the control group (two occlusions, one perforation, and one pulmonary embolism). After first disease progression, no substantial differences in the incidence of grade 3 or 4 adverse events were reported between the control and experimental groups, with the exception of neurotoxicity, which was only reported in the experimental group (six [5%] of 132 patients). Serious adverse events after disease progression occurred in 20 (15%) patients in the experimental group and 25 (12%) in the control group. Three treatment-related deaths after first disease progression were reported in the experimental group (two intestinal occlusions and one sepsis) and four in the control group (one intestinal occlusion, one intestinal perforation, one cerebrovascular event, and one sepsis).
Interpretation
Upfront FOLFOXIRI plus bevacizumab followed by the reintroduction of the same regimen after disease progression seems to be a preferable therapeutic strategy to sequential administration of chemotherapy doublets, in combination with bevacizumab, for patients with metastatic colorectal cancer selected according to the study criteria.
Funding
The GONO Cooperative Group, the ARCO Foundation, and F Hoffmann–La Roche.
中文翻译:

与转移性结直肠癌(TRIBE2)的患者相比,前期FOLFOXIRI加贝伐单抗和进展后重新引入与mFOLFOX6加贝伐单抗再由FOLFIRI加贝伐单抗治疗:一项多中心,开放标签,3期,随机对照试验。
背景
三联体FOLFOXIRI(氟尿嘧啶,亚叶酸钙,奥沙利铂和伊立替康)加贝伐单抗与转移性结直肠癌患者相比,FOLFIRI(氟尿嘧啶,亚叶酸钙和伊立替康)加贝伐单抗显示出更好的结局。但是,与预先计划的双重治疗顺序策略相比,预先暴露于三种细胞毒性药物的实际益处尚不清楚,而且疾病进展后治疗的可行性或功效也不清楚。我们的目的是比较疾病进展后的前期FOLFOXIRI的预先计划策略,然后与采用mFOLFOX6序列(氟尿嘧啶,亚叶酸和奥沙利铂)和FOLFIRI doublet以及贝伐单抗联合应用相同的方案。
方法
TRIBE2是一项开放标签的3期随机研究,研究对象为18-75岁的东部合作肿瘤小组(ECOG)表现状态为2,无法切除,先前未治疗的转移性结直肠癌,来自58个意大利肿瘤学部门。根据中心,ECOG表现状态,原发肿瘤位置和既往辅助化疗对患者进行分层。结合最小化算法的随机化系统用于通过基于网络的屏蔽分配程序将患者(1:1)随机分配给两种不同的治疗策略。在对照组中,患者在120分钟内接受一线mFOLFOX6(85 mg / m 2静脉注射奥沙利铂和200 mg / m 2的亚叶酸钙; 400 mg / m 2静脉内推注氟尿嘧啶;2400 mg / m 2的氟尿嘧啶连续输注48小时)+贝伐单抗(30分钟内静脉滴注5 mg / kg),然后FOLFIRI(120分钟内180 mg / m 2静脉注射依立替康和200 mg / m 2亚叶酸钙同时输注;疾病进展后,静脉内推注400 mg / m 2氟尿嘧啶;持续2 h持续输注2400 mg / m 2氟尿嘧啶48小时)加贝伐单抗。在实验组中,患者在60分钟内接受FOLFOXIRI(165 mg / m 2静脉注射伊立替康; 85 mg / m 2静脉内奥沙利铂与200 mg / m 2的亚叶酸钙在120分钟内同时给药; 3200 mg / m 2持续输注氟尿嘧啶48小时)加贝伐单抗,然后在病情恶化后重新引入相同的治疗方案。每14天重复组合治疗多达8个周期,然后进行氟尿嘧啶和亚叶酸钙蛋白(在最后一个诱导周期以相同剂量给药)加贝伐单抗维持治疗,直到疾病进展,不可接受的不良事件或同意撤消。患者和研究者没有被掩盖。主要终点是无进展生存期2,无进展生存期2定义为从第一个疾病进展或死亡后进行的任何治疗中随机分配到疾病进展的时间,并通过治疗意图进行了分析。在接受至少一剂指定治疗剂量的患者中评估安全性。研究募集工作已经完成,后续工作正在进行中。
发现
在2015年2月26日至2017年5月15日之间,随机分配了679例患者并接受治疗(对照组340例,实验组339例)。截至数据截止(2019年7月30日),中位随访时间为35·9个月(IQR 30·1-41-4·4)。实验组中位无进展生存期2为19·2个月(95%CI 17·3-21·4),对照组为16·4个月(15·1-17.5)(危险比[HR ] 0·74,95%CI 0·63-0·88; p = 0·0005)。一线治疗期间,最常见的3-4级全因事件是腹泻(57 [17%] vs 18 [5%]),中性粒细胞减少症(168 [50%] vs 71 [21%]),和动脉高血压(25 [7%] vs35 [10%]),实验组与对照组比较。在实验组中有84位(25%)患者发生严重不良事件,在对照组中有56位(17%)患者发生严重不良事件。实验组报告8例与治疗相关的死亡(2例肠梗阻,2例肠穿孔,2个败血症,1例心肌梗塞和1例出血),对照组4例死亡(2例梗塞,1例穿孔和1例肺栓塞)。在首次疾病进展后,对照组和实验组之间报告的3级或4级不良事件的发生率没有实质性差异,只有神经毒性例外,仅在实验组中报告(132名患者中的六名[5%]) )。实验组中有20(15%)名患者发生了疾病进展后的严重不良事件,而对照组中有25名(12%)。在实验组中报告了三例首次疾病进展后与治疗相关的死亡(两个肠梗阻和一个败血症),在对照组中报告了四例(一个肠梗阻,一个肠穿孔,一个脑血管事件和一个败血症)。
解释
对于根据研究标准选择的转移性结直肠癌患者,预后的FOLFOXIRI加贝伐单抗随后在疾病进展后重新引入相同的方案似乎是先后顺序应用化疗双联联合贝伐单抗的首选治疗策略。
资金
GONO合作小组,ARCO基金会和F Hoffmann-La Roche。











































 京公网安备 11010802027423号
京公网安备 11010802027423号