当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A synergistic synthetic and computational insights towards anomerization of N-Nitro pyrimidine nucleosides using fluorinating agents
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jfluchem.2020.109504 Ahmed Khalil , Imene Bayach , Christophe Mathé
中文翻译:

使用氟化剂对N-硝基嘧啶核苷进行异构化的协同合成和计算见解
更新日期:2020-03-10
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jfluchem.2020.109504 Ahmed Khalil , Imene Bayach , Christophe Mathé
|
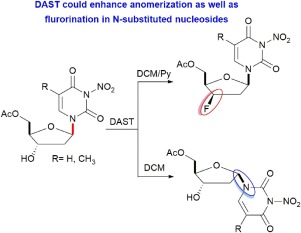
DAST and Deoxofluor are usually used for nucleophilic fluorination of nucleosides via SN1 or SN2 mechanism. DAST and Deoxo-fluor could enhance anomerization of N-substituted thymidine and 2'-deoxyuridine in dichloromethane into the more stable and favored α-anomers due to in-situ liberation of HF. This is strongly supported by computational calculations based on the density functional theory, that were performed to rationalize energy stability and electronic properties of both anomers in order to provide further insights into the proposed mechanism.
中文翻译:

使用氟化剂对N-硝基嘧啶核苷进行异构化的协同合成和计算见解
DAST和脱氧氟通常通过S N 1或S N 2机制用于核苷的亲核氟化。DAST和Deoxo-fluor由于HF的原位释放,可增强二氯甲烷中N-取代的胸苷和2'-脱氧尿苷的异构化成更稳定和更有利的α-端基异构体。这是由基于密度泛函理论的计算计算强有力地支持的,该计算计算是为了合理化两种端基异构体的能量稳定性和电子性质,以便为提出的机理提供进一步的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号