当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bacterioruberin from Haloarchaea plus dexamethasone in ultra-small macrophage-targeted nanoparticles as potential intestinal repairing agent.
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.colsurfb.2020.110961 Leticia Herminia Higa 1 , Priscila Schilrreff 1 , Andrés Martín Briski 1 , Horacio Emanuel Jerez 1 , Marcelo Alexandre de Farias 2 , Rodrigo Villares Portugal 2 , Eder Lilia Romero 1 , Maria Jose Morilla 1
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.colsurfb.2020.110961 Leticia Herminia Higa 1 , Priscila Schilrreff 1 , Andrés Martín Briski 1 , Horacio Emanuel Jerez 1 , Marcelo Alexandre de Farias 2 , Rodrigo Villares Portugal 2 , Eder Lilia Romero 1 , Maria Jose Morilla 1
Affiliation
|
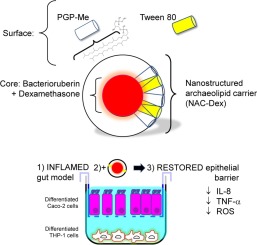
Oral administration of antioxidant and anti-inflammatory drugs have the potential to improve the current therapy of inflammatory bowel disease. Success of oral treatments, however, depends on the capacity of drugs to remain structurally stable along the gastrointestinal tract, and on the feasibility of accessing the target cells. Delivering anti-inflammatory and antioxidant drugs to macrophages using targeted nanoparticles, could make treatments more efficient. In this work structural features and in vitro activity of macrophage-targeted nanostructured archaeolipid carriers (NAC) containing the high antioxidant dipolar C50 carotenoid bacterioruberin (BR) plus dexamethasone (Dex): NAC-Dex, are described. Ultra-small (66 nm), -32 mV ζ potential, 1200 μg Dex /ml NAC-Dex, consisted of a compritol and BR core, covered by a shell of sn 2,3 ether linked archaeolipids and Tween 80 (2: 2: 1.2: 3 % w/w) were obtained. NAC-Dex were extensively captured by macrophages and Caco-2 cells and displayed high anti-inflammatory and antioxidant activities on a gut inflammation model made of Caco-2 cells and lipopolysaccharide stimulated THP-1 derived macrophages reducing 65 % and 55 % TNF-α and IL-8 release, respectively and 60 % reactive oxygen species production. NAC-Dex also reversed the morphological changes induced by inflammation and increased the transepithelial electrical resistance, partly reconstituting the barrier function. Activity of BR and Dex in NAC-Dex was partially protected after simulated gastrointestinal digestion, improving the chances of BR-Dex joint activity. Results suggest that oral NAC-Dex deserve further exploration as intestinal barrier repairing agent.
中文翻译:

来自Haloarchaea的芽孢杆菌素加地塞米松在超小巨噬细胞靶向纳米颗粒中作为潜在的肠道修复剂。
口服抗氧化剂和消炎药具有改善当前炎症性肠病治疗的潜力。但是,口服治疗的成功取决于药物沿胃肠道保持结构稳定的能力,以及进入靶细胞的可行性。使用靶向的纳米颗粒向巨噬细胞递送抗炎和抗氧化药物可以使治疗更有效。在这项工作中,描述了以巨噬细胞为靶标的纳米结构古脂质载体(NAC)的结构特征和体外活性,该载体包含高抗氧化剂偶极C50类胡萝卜素细菌素(BR)和地塞米松(Dex):NAC-Dex。超小型(66 nm),-32 mVζ电位,1200μgDex / ml NAC-Dex,由compritol和BR核组成,被sn 2的壳覆盖 得到3个醚连接的古脂质和吐温80(2∶2∶1.2∶3%w / w)。NAC-Dex被巨噬细胞和Caco-2细胞广泛捕获,并在由Caco-2细胞和脂多糖刺激的THP-1衍生的巨噬细胞组成的肠道炎症模型上显示出较高的抗炎和抗氧化活性,从而减少了65%和55%的TNF-α IL-8和IL-8分别释放和60%的活性氧产生。NAC-Dex还可以逆转由炎症引起的形态变化,并增加跨上皮电阻,从而部分重新构建屏障功能。模拟胃肠道消化后,NAC-Dex中BR和Dex的活性得到部分保护,从而提高了BR-Dex关节活性的机会。结果表明,口服NAC-Dex作为肠屏障修复剂值得进一步探索。
更新日期:2020-03-10
中文翻译:

来自Haloarchaea的芽孢杆菌素加地塞米松在超小巨噬细胞靶向纳米颗粒中作为潜在的肠道修复剂。
口服抗氧化剂和消炎药具有改善当前炎症性肠病治疗的潜力。但是,口服治疗的成功取决于药物沿胃肠道保持结构稳定的能力,以及进入靶细胞的可行性。使用靶向的纳米颗粒向巨噬细胞递送抗炎和抗氧化药物可以使治疗更有效。在这项工作中,描述了以巨噬细胞为靶标的纳米结构古脂质载体(NAC)的结构特征和体外活性,该载体包含高抗氧化剂偶极C50类胡萝卜素细菌素(BR)和地塞米松(Dex):NAC-Dex。超小型(66 nm),-32 mVζ电位,1200μgDex / ml NAC-Dex,由compritol和BR核组成,被sn 2的壳覆盖 得到3个醚连接的古脂质和吐温80(2∶2∶1.2∶3%w / w)。NAC-Dex被巨噬细胞和Caco-2细胞广泛捕获,并在由Caco-2细胞和脂多糖刺激的THP-1衍生的巨噬细胞组成的肠道炎症模型上显示出较高的抗炎和抗氧化活性,从而减少了65%和55%的TNF-α IL-8和IL-8分别释放和60%的活性氧产生。NAC-Dex还可以逆转由炎症引起的形态变化,并增加跨上皮电阻,从而部分重新构建屏障功能。模拟胃肠道消化后,NAC-Dex中BR和Dex的活性得到部分保护,从而提高了BR-Dex关节活性的机会。结果表明,口服NAC-Dex作为肠屏障修复剂值得进一步探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号