Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.bbapap.2020.140410 Seongmin Ha 1 , Tadatoshi Kinouchi 2 , Noriko Fujii 3
|
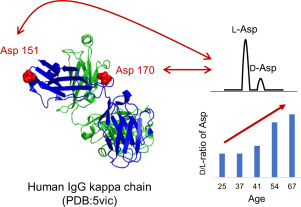
Isomerization of aspartate (Asp) is a common non-enzymatic posttranslational modification. Isomerized residues accumulate in proteins associated with age-related human disorders such as cataract and are well known to affect protein structure and function. We previously detected d-Asp-containing peptides in human serum. In this study, we investigated whether isomerized Asp residues are present in human immunoglobulin G (IgG) kappa chain by a qualitative d-amino acid analysis based on diastereomer formation and liquid chromatography tandem mass spectrometry (LC-MS/MS). We also investigated the d/l ratio of Asp residues in the IgG kappa chain in serum from donors aged 25, 37, 41, 54 and 67 years. As a result, two isomerized Asp residues, Asp151 and Asp170, were detected in the IgG kappa chain, and the d/l ratio of these residues was found to increase with aging. To assess the effects of this isomerization, we synthesized four isomeric peptides of IgG kappa chain containing lα-, lβ-, dα-, or dβ-Asp at position 170, and compared their secondary structures by CD spectroscopy. Peptide containing normal lα-Asp170 showed type II β-turn structure, while the other isomeric peptides showed random structure, clearly indicating that substitution of a single Asp isomer alters the secondary structure of the peptide. Because IgG is a main component of humoral immunity, Asp isomerization in IgG may reflect changes of structure and decrease in immune function. Proteome research on serum from the standpoint of racemization might enable us to develop new kinds of biomarker and new directions to study the aging process.
中文翻译:

人免疫球蛋白Gκ链中Asp的年龄相关异构化。
天冬氨酸(Asp)的异构化是常见的非酶翻译后修饰。异构化的残基积累在与年龄相关的人类疾病(如白内障)相关的蛋白质中,众所周知会影响蛋白质的结构和功能。我们先前在人血清中检测到了含d -Asp的肽。在这项研究中,我们通过基于非对映异构体形成和液相色谱串联质谱(LC-MS / MS)的定性d-氨基酸分析,研究了人类免疫球蛋白G(IgG)κ链中是否存在异构化的Asp残基。我们还研究了d / l年龄分别为25、37、41、54和67岁的供体的血清IgGκ链中Asp残基的比率。结果,在IgGκ链中检测到两个异构化的Asp残基Asp151和Asp170,并且发现这些残基的d / l比随着老化而增加。为了评估此异构化的影响,我们合成了含IgGκ链四种肽异构体升α-,升β-,d α-,或d β -天冬氨酸在170位置,并通过比较CD光谱的二级结构。含有正常l的肽α-Asp170显示II型β转角结构,而其他异构肽显示随机结构,清楚地表明单个Asp异构体的取代会改变该肽的二级结构。由于IgG是体液免疫的主要成分,因此IgG中的Asp异构化可能反映结构的变化和免疫功能的降低。从外消旋的角度对血清进行蛋白质组学研究可能使我们能够开发出新的生物标志物,并为研究衰老过程提供新的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号