Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.comptc.2020.112780 Tao Zhou , Li Ma , Hongshan Chen
|
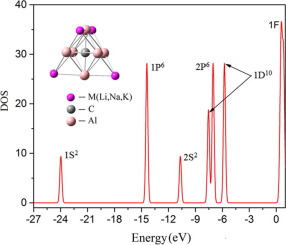
The stabilities and electronic structures of Al6CMn (M=Li, Na, K; n=2,4,6) are studied by evolutionary algorithm combined with ab initio methods. The strong attraction of the C4+ core to the valence electrons makes the 2S state considerably lower and the degenerated 2P6 being located in-between the split 1D10 states, so the 26 valence electrons form closed 1S21P62S21D102P6 shells. C-doping enhances considerably the stabilities of metal clusters. In Al6CMn clusters, the Al6Cq- with nearly all the valence electrons form Zintl anions and the M+ cations are ionically bonded on the peripheries. As Li+ has smallest radius, Al6CLi4 possesses strongest thermal and chemical stabilities. The electronic structure of Al6C4- accords with the 8+18-electron rule and the Wade-Mingos rule, and can be regarded as a superatomic anion with specific stability. It can be used as building blocks to form Zintl phase structures by combining with metal counterparts.
中文翻译:

Al 6 CM n(M = Li,Na,K; n = 2,4,6)团簇的电子结构和稳定性
通过进化算法结合从头算方法研究了Al 6 CM n(M = Li,Na,K; n = 2,4,6)的稳定性和电子结构。C 4+核对价电子的强大吸引力使2S状态大大降低,并且退化的2P 6位于分裂的1D 10状态之间,因此26个价电子形成闭合的1S 2 1P 6 2S 2 1D 10 2P 6壳。C掺杂大大提高了金属团簇的稳定性。在Al 6 CM n团簇中,Al 6 C q-几乎所有的价电子都形成Zintl阴离子,并且M +阳离子通过离子键结合在外围。由于Li +的半径最小,因此Al 6 CLi 4具有最强的热稳定性和化学稳定性。Al 6 C 4-的电子结构符合8 + 18电子规则和Wade-Mingos规则,可以看作是具有特定稳定性的超原子阴离子。通过与金属配合物结合,可以用作构建Zintl相结构的基础。










































 京公网安备 11010802027423号
京公网安备 11010802027423号