Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Genome-wide ER-phagy Screen Highlights Key Roles of Mitochondrial Metabolism and ER-Resident UFMylation.
Cell ( IF 45.5 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.cell.2020.02.017 Jin Rui Liang 1 , Emily Lingeman 2 , Thao Luong 2 , Saba Ahmed 2 , Matthias Muhar 3 , Truc Nguyen 4 , James A Olzmann 5 , Jacob E Corn 1
Cell ( IF 45.5 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.cell.2020.02.017 Jin Rui Liang 1 , Emily Lingeman 2 , Thao Luong 2 , Saba Ahmed 2 , Matthias Muhar 3 , Truc Nguyen 4 , James A Olzmann 5 , Jacob E Corn 1
Affiliation
|
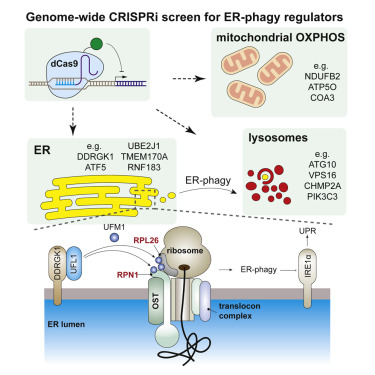
Selective autophagy of organelles is critical for cellular differentiation, homeostasis, and organismal health. Autophagy of the ER (ER-phagy) is implicated in human neuropathy but is poorly understood beyond a few autophagosomal receptors and remodelers. By using an ER-phagy reporter and genome-wide CRISPRi screening, we identified 200 high-confidence human ER-phagy factors. Two pathways were unexpectedly required for ER-phagy. First, reduced mitochondrial metabolism represses ER-phagy, which is opposite of general autophagy and is independent of AMPK. Second, ER-localized UFMylation is required for ER-phagy to repress the unfolded protein response via IRE1α. The UFL1 ligase is brought to the ER surface by DDRGK1 to UFMylate RPN1 and RPL26 and preferentially targets ER sheets for degradation, analogous to PINK1-Parkin regulation during mitophagy. Our data provide insight into the cellular logic of ER-phagy, reveal parallels between organelle autophagies, and provide an entry point to the relatively unexplored process of degrading the ER network.
中文翻译:

全基因组的ER噬菌体筛选突出显示了线粒体代谢和ER驻留UFMylation的关键作用。
细胞器的选择性自噬对于细胞分化,体内平衡和机体健康至关重要。ER的自噬(ER-phagy)与人类神经病有关,但除少数自噬体受体和重塑剂外,人们对其了解甚少。通过使用ER-吞噬记者和全基因组CRISPRi筛选,我们鉴定了200个高可信度的人类ER-吞噬因子。ER吞噬意外地需要两个途径。首先,线粒体代谢减少会抑制ER吞噬,这与一般自噬相反,并且独立于AMPK。其次,ER吞噬需要通过ER定位的UFMylation来抑制通过IRE1α展开的蛋白质反应。DDRGK1将UFL1连接酶带到ER表面,到达UFMylate RPN1和RPL26,并优先靶向ER薄片进行降解,类似于线粒吞噬过程中的PINK1-Parkin调节。
更新日期:2020-03-10
中文翻译:

全基因组的ER噬菌体筛选突出显示了线粒体代谢和ER驻留UFMylation的关键作用。
细胞器的选择性自噬对于细胞分化,体内平衡和机体健康至关重要。ER的自噬(ER-phagy)与人类神经病有关,但除少数自噬体受体和重塑剂外,人们对其了解甚少。通过使用ER-吞噬记者和全基因组CRISPRi筛选,我们鉴定了200个高可信度的人类ER-吞噬因子。ER吞噬意外地需要两个途径。首先,线粒体代谢减少会抑制ER吞噬,这与一般自噬相反,并且独立于AMPK。其次,ER吞噬需要通过ER定位的UFMylation来抑制通过IRE1α展开的蛋白质反应。DDRGK1将UFL1连接酶带到ER表面,到达UFMylate RPN1和RPL26,并优先靶向ER薄片进行降解,类似于线粒吞噬过程中的PINK1-Parkin调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号