当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.03.006 Zhipeng Liu 1 , Yang Zhang 2 , Sarah Graham 3 , Xiaokun Wang 2 , Defeng Cai 4 , Menghao Huang 5 , Roger Pique-Regi 6 , Xiaocheng Charlie Dong 5 , Y Eugene Chen 3 , Cristen Willer 7 , Wanqing Liu 8
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jhep.2020.03.006 Zhipeng Liu 1 , Yang Zhang 2 , Sarah Graham 3 , Xiaokun Wang 2 , Defeng Cai 4 , Menghao Huang 5 , Roger Pique-Regi 6 , Xiaocheng Charlie Dong 5 , Y Eugene Chen 3 , Cristen Willer 7 , Wanqing Liu 8
Affiliation
|
BACKGROUND & AIMS
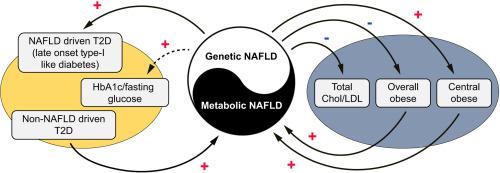
Non-alcoholic fatty liver disease (NAFLD), type 2 diabetes (T2D) and obesity are epidemiologically correlated with each other but the causal inter-relationships among them remains incompletely understood. We aim to explore the causal relationships among the three diseases. METHODS
Using both UK BioBank and the largest-to-date publicly available GWAS data, we performed a two-sample bidirectional Mendelian Randomization (MR) analysis to test the causal inter-relationships among NAFLD, T2D, and obesity. Transgenic mice expressing the human PNPLA3-I148M isoforms (TghPNPLA3-I148M) were used as an example to validate the causal effects and explore the underlying mechanisms. RESULTS
Genetically-instrumented NAFLD significantly increased the risk for T2D and central obesity but not insulin resistance or generalized obesity, while genetically-driven T2D, BMI and WHRadjBMI causally increase the NAFLD risk. The animal study focusing on PNPLA3 well-corroborated these causal effects: compared to the TghPNPLA3-I148I controls, the TghPNPLA3-I148M mice developed glucose intolerance and increased visceral fat, but normal insulin sensitivity, reduced body weight, and decreased circulating total cholesterol. Mechanistically, the TghPNPLA3-I148M mice demonstrated decreased pancreas insulin but increased glucagon secretion, which was associated with increased pancreatic inflammation. There were significantly suppressed transcription of hepatic cholesterol biosynthesis pathway genes while activation of thermogenic pathway genes in subcutaneous and brown adipose tissues but not in visceral fat of the TghPNPLA3-I148M mice compared to the TghPNPLA3-I148I mice. CONCLUSIONS
Our study suggests that lifelong, genetically-driven NAFLD causally promotes T2D with a late onset Type-1 like diabetic subphenotype and central obesity; while genetically-driven T2D, obesity, and central obesity all causally increase the NAFLD risk. This causal relationship revealed new insights into the nature and nurture of these diseases and provided novel hypotheses for disease subphenotyping.
中文翻译:

NAFLD、T2D 和肥胖之间的因果关系对疾病亚表型分析具有影响
背景和目的非酒精性脂肪肝病 (NAFLD)、2 型糖尿病 (T2D) 和肥胖在流行病学上相互关联,但它们之间的因果关系仍不完全清楚。我们的目的是探讨这三种疾病之间的因果关系。方法 使用英国生物银行和迄今为止最大的公开可用的 GWAS 数据,我们进行了双样本双向孟德尔随机化 (MR) 分析,以测试 NAFLD、T2D 和肥胖之间的因果关系。以表达人 PNPLA3-I148M 亚型(TghPNPLA3-I148M)的转基因小鼠为例,验证因果效应并探索潜在机制。结果 基因检测的 NAFLD 显着增加了 T2D 和中心性肥胖的风险,但不会增加胰岛素抵抗或全身性肥胖的风险,而基因驱动的 T2D、BMI 和 WHRadjBMI 会因果性地增加 NAFLD 风险。专注于 PNPLA3 的动物研究很好地证实了这些因果效应:与 TghPNPLA3-I148I 对照相比,TghPNPLA3-I148M 小鼠出现葡萄糖耐受不良和内脏脂肪增加,但胰岛素敏感性正常,体重减轻,循环总胆固醇减少。从机制上讲,TghPNPLA3-I148M 小鼠表现出胰腺胰岛素减少,但胰高血糖素分泌增加,这与胰腺炎症增加有关。与 TghPNPLA3-I148I 小鼠相比,TghPNPLA3-I148M 小鼠的皮下和棕色脂肪组织中的肝胆固醇生物合成途径基因的转录显着受到抑制,而生热途径基因的激活则没有在内脏脂肪中的激活。结论 我们的研究表明,基因驱动的终生 NAFLD 会促进 T2D 的发生,并伴有迟发的 1 型糖尿病亚表型和向心性肥胖;而基因驱动的 T2D、肥胖和中心性肥胖都会增加 NAFLD 的风险。这种因果关系揭示了对这些疾病的本质和培养的新见解,并为疾病亚表型分析提供了新的假设。
更新日期:2020-08-01
中文翻译:

NAFLD、T2D 和肥胖之间的因果关系对疾病亚表型分析具有影响
背景和目的非酒精性脂肪肝病 (NAFLD)、2 型糖尿病 (T2D) 和肥胖在流行病学上相互关联,但它们之间的因果关系仍不完全清楚。我们的目的是探讨这三种疾病之间的因果关系。方法 使用英国生物银行和迄今为止最大的公开可用的 GWAS 数据,我们进行了双样本双向孟德尔随机化 (MR) 分析,以测试 NAFLD、T2D 和肥胖之间的因果关系。以表达人 PNPLA3-I148M 亚型(TghPNPLA3-I148M)的转基因小鼠为例,验证因果效应并探索潜在机制。结果 基因检测的 NAFLD 显着增加了 T2D 和中心性肥胖的风险,但不会增加胰岛素抵抗或全身性肥胖的风险,而基因驱动的 T2D、BMI 和 WHRadjBMI 会因果性地增加 NAFLD 风险。专注于 PNPLA3 的动物研究很好地证实了这些因果效应:与 TghPNPLA3-I148I 对照相比,TghPNPLA3-I148M 小鼠出现葡萄糖耐受不良和内脏脂肪增加,但胰岛素敏感性正常,体重减轻,循环总胆固醇减少。从机制上讲,TghPNPLA3-I148M 小鼠表现出胰腺胰岛素减少,但胰高血糖素分泌增加,这与胰腺炎症增加有关。与 TghPNPLA3-I148I 小鼠相比,TghPNPLA3-I148M 小鼠的皮下和棕色脂肪组织中的肝胆固醇生物合成途径基因的转录显着受到抑制,而生热途径基因的激活则没有在内脏脂肪中的激活。结论 我们的研究表明,基因驱动的终生 NAFLD 会促进 T2D 的发生,并伴有迟发的 1 型糖尿病亚表型和向心性肥胖;而基因驱动的 T2D、肥胖和中心性肥胖都会增加 NAFLD 的风险。这种因果关系揭示了对这些疾病的本质和培养的新见解,并为疾病亚表型分析提供了新的假设。







































 京公网安备 11010802027423号
京公网安备 11010802027423号