European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.ejmech.2020.112219 Vikas Sharma 1 , Rajiv Kumar 2 , Andrea Angeli 3 , Claudiu T Supuran 3 , Pawan K Sharma 4
|
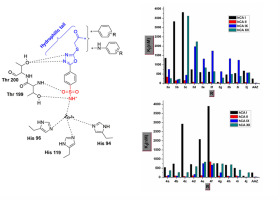
Two new series of 1,3,4-oxadiazole benzenesulfonamide hybrids 3 and 4, having twenty novel compounds, have been designed and synthesized in order to assess their inhibition potential as CAIs against hCA I, II, IX, and XII. ‘Tail approach’ strategy has been used to design the aromatic sulfonamide scaffolds with carbonyl and amide linker. Excellent inhibitory activity against hCA I has been exhibited by compounds 3g and 4j, 3.5 magnitude of order better than reference drug AAZ (KI = 250 nM). Moreover, compound 4j (KI = 7.9 nM) effectively inhibited glaucoma-associated hCA II isoform as well as tumor-associated hCA IX isoform with KI = 16.3 nM. Further hCA XII was weakly inhibited by all the compounds with KI values ranging from 0.23 μM to 3.62 μM. Interestingly structure-activity relationship (SAR) study indicates that N-(3-nitrophenyl)-2-((5-(4-sulfamoylphenyl)-1,3,4-oxadiazol-2-yl)thio)acetamide (4j) is a potent compound to be investigated further for antiglaucoma and antitumor activity. The chemistry of the nature of different substitutions on the 1,3,4-oxadiazole bearing benzenesulfonamide substituted aromatic ring for potency and selectivity over one hCA isoform versus others is deliberated in the present study. In this context, the 1,3,4-oxadiazole motif can be a valuable tool worth developing for the procurement of novel and potent selective CAIs potentially useful for the management of a variety of diseases as chemotherapeutic agents.
中文翻译:

尾部法合成新型苯磺酰胺,包含 1,3,4-恶二唑杂化物作为碳酸酐酶 I、II、IX 和 XII 同工酶的有效抑制剂
设计和合成了两个新系列的 1,3,4-恶二唑苯磺酰胺杂化物3和4,具有 20 种新化合物,以评估它们作为 CAI 对 hCA I、II、IX 和 XII 的抑制潜力。 “尾部方法”策略已用于设计具有羰基和酰胺连接基的芳香族磺酰胺支架。化合物3g和4j对hCA I表现出优异的抑制活性,比参考药物AAZ好3.5个数量级(K I = 250 nM)。此外,化合物4j (K I = 7.9 nM) 有效抑制青光眼相关的 hCA II 亚型以及肿瘤相关的 hCA IX 亚型,K I = 16.3 nM。此外,所有化合物均对 hCA XII 产生微弱抑制,K I值范围为 0.23 μM 至 3.62 μM。有趣的是构效关系 (SAR) 研究表明 N-(3-硝基苯基)-2-((5-(4-氨磺酰基苯基)-1,3,4-恶二唑-2-基)硫代)乙酰胺 ( 4j ) 是一种有效的化合物,有待进一步研究其抗青光眼和抗肿瘤活性。本研究探讨了带有苯磺酰胺的 1,3,4-恶二唑取代的芳环上不同取代基的性质对一种 hCA 异构体相对于其他异构体的效力和选择性的化学性质。在这种情况下,1,3,4-恶二唑基序可能是一个值得开发的有价值的工具,用于采购新颖且有效的选择性 CAI,这些 CAI 可能可用于作为化疗药物来管理多种疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号