Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.apcata.2020.117515 Philip Anggo Krisbiantoro , Tomokazu Togawa , Lina Mahardiani , Haruka Aihara , Ryoichi Otomo , Yuichi Kamiya
|
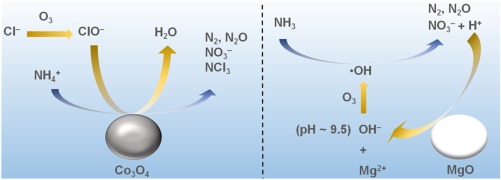
In this study, the reaction mechanisms for ozonation of ammonia nitrogen in the presence of Co3O4 or MgO were investigated. For the reaction over Co3O4, Cl– in the reaction solution was indispensable and ClO– was formed by a non-catalytic oxidation of Cl–. Co3O4 promoted the reaction of NH4+ with ClO– to give the products including NO3–, chloramines and gaseous products. In contrast, Cl– was unnecessary for the reaction with MgO. pH of the reaction solution was maintained at around 9 throughout the reaction owing to partial dissolution of MgO. Ammonia nitrogen was decomposed to mainly NO3– by non-catalytic radical reaction involving OH·, which was formed by the reaction of OH– with O3 in weakly basic solution. To keep the reaction solution weakly basic, H+ formed with the decomposition of NH4+ was neutralized. As a result, about the same amount of Mg2+ as that of decomposed ammonia nitrogen was dissolved.
中文翻译:

氧化钴或氧化镁在水中氨氮臭氧化中的作用
在这项研究中,研究了在Co 3 O 4或MgO存在下氨氮臭氧化的反应机理。对于在Co 3 O 4上进行的反应,反应溶液中的Cl–是必不可少的,而ClO–是由Cl–的非催化氧化形成的。Co 3 O 4促进了NH 4 +与ClO–的反应,生成包括NO 3–,氯胺和气态产物的产物。相反,Cl–对于与MgO的反应是不必要的。由于MgO的部分溶解,在整个反应过程中反应溶液的pH保持在9左右。氨氮主要分解为NO 3–通过涉及OH·的非催化自由基反应,这是由OH–与O 3在弱碱性溶液中反应形成的。保持反应溶液的弱碱性,H +与NH分解形成4 +中和。结果,溶解了与分解的氨氮大致相同量的Mg 2+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号