当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalyzed asymmetric cascade Mannich/cyclization of 3-isothiocyanato oxindoles with cyclic ketimines for the synthesis of polycyclic spiro-thioimidazolidine-oxindoles
Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.tet.2020.131116 Chuan-Bao Zhang , Pei-Hao Dou , Jian-Qiang Zhao , Wei-Cheng Yuan
中文翻译:

有机催化的不对称级联曼尼希/ 3-异硫氰酸根合吲哚与环酮亚胺的环化反应,用于合成多环螺硫代咪唑烷-吲哚
更新日期:2020-03-10
Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.tet.2020.131116 Chuan-Bao Zhang , Pei-Hao Dou , Jian-Qiang Zhao , Wei-Cheng Yuan
|
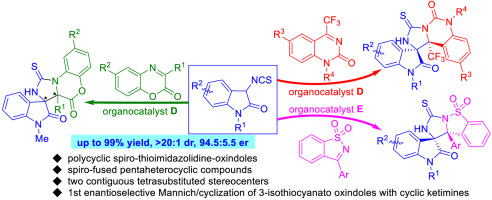
The first diastereo- and enantioselective cascade Mannich/cyclization reaction of 3-isothiocyanato oxindoles with cyclic ketimines has been successfully developed. By using chiral bifunctional organocatalysts, a range of structurally diverse chiral polycyclic spiro-thioimidazolidine-oxindoles, bearing two contiguous tetrasubstituted stereocenters, could be obtained in excellent yields (up to 99%) with good diastereo- and enantioselectivities (up to >20:1 dr and 94.5:5.5 er) under mild reaction conditions.
中文翻译:

有机催化的不对称级联曼尼希/ 3-异硫氰酸根合吲哚与环酮亚胺的环化反应,用于合成多环螺硫代咪唑烷-吲哚
已成功开发了3-异硫氰酸根合吲哚与环状酮亚胺的第一个非对映和对映选择性级联曼尼希/环化反应。通过使用手性双功能有机催化剂,可以以优异的收率(高达99%),良好的非对映和对映选择性(高达> 20:1)获得一系列带有两个连续四取代立体中心的结构多样的手性多环螺-硫代咪唑烷-氧吲哚。 dr和94.5:5.5 er)在温和的反应条件下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号