Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.apcatb.2020.118874 Hongxiang Zhang , Chenwei Li , Lai Lyu , Chun Hu
|
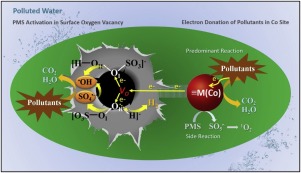
Peroxymonosulfate (PMS) activation in heterogeneous processes for pollutant degradation is a promising water purification technology. However, the existed rate limiting step greatly restrains its performance and increases the consumption of PMS and energy. Herein, we offer a new strategy to solve this problem. In this work, surface oxygen vacancy (VO)-rich ZnFe0.8Co0.4O2.4 nanoparticles were prepared and characterized, which exhibited high activity and stability for refractory pollutant degradation with PMS activation. It was found that PMS ([O3S-OI-OII-H]−) could be adsorbed and trapped by the surface oxygen vacancies in the form of OI-Vo or OII-Vo during the reaction. Different electron transfer pathways from Vo to different O sites of PMS was realized in the solid-liquid interface based on the generation of •OH, SO4•− or H2 from PMS reduction. Pollutants were predominantly adsorbed at metal Co sites in which their electrons were captured by metal species and then transferred to the surface oxygen vacancies, achieving efficient recycling of electrons in the aqueous suspensions. This system achieves a dual-pathway degradation of pollutants and electron transfer from pollutants to PMS to produce free radicals and H2, essentially changing the traditional concepts of pollutant removal and providing a sustainable strategy for pollutant utilization during water purification.
中文翻译:

表面氧空位通过钴锌铁氧体上电子给体污染物的电子捐赠而诱导过氧单硫酸盐活化以进行水净化
在异质过程中过氧单硫酸盐(PMS)活化用于污染物降解是一种有前途的水净化技术。但是,现有的限速步骤大大限制了它的性能,增加了PMS和能源的消耗。在此,我们提供了解决这一问题的新策略。在这项工作中,表面氧空位(V ö)丰富的ZnFe 0.8钴0.4 ø 2.4纳米颗粒的制备和表征,其显示出高的活性和稳定性与PMS激活耐火污染物的降解。发现PMS([O 3 S-O I -O II -H] -)在反应过程中可能以O I - Vo或O II - Vo的形式被表面氧空位吸附和捕获。基于• OH,SO 4 •-或H 2的生成,在固液界面上实现了从Vo到PMS的不同O位的不同电子转移途径。减少PMS。污染物主要吸附在金属物种的电子被金属Co捕获的位置,然后转移到表面的氧空位,从而实现了水悬浮液中电子的有效循环。该系统实现了污染物的双重途径降解以及电子从污染物到PMS的转移,从而产生自由基和H 2,从根本上改变了传统的污染物去除概念,并为水净化过程中的污染物利用提供了可持续的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号