当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Positive Charge in the Outer Coordination Sphere of an Artificial Enzyme Increases CO2 Hydrogenation
Organometallics ( IF 2.5 ) Pub Date : 2020-03-10 , DOI: 10.1021/acs.organomet.9b00843 Joseph A. Laureanti 1 , Bojana Ginovska 1 , Garry W. Buchko 2, 3 , Gregory K. Schenter 1 , Margaret Hebert 1 , Oleg A. Zadvornyy 4 , John W. Peters 1, 4 , Wendy J. Shaw 1
Organometallics ( IF 2.5 ) Pub Date : 2020-03-10 , DOI: 10.1021/acs.organomet.9b00843 Joseph A. Laureanti 1 , Bojana Ginovska 1 , Garry W. Buchko 2, 3 , Gregory K. Schenter 1 , Margaret Hebert 1 , Oleg A. Zadvornyy 4 , John W. Peters 1, 4 , Wendy J. Shaw 1
Affiliation
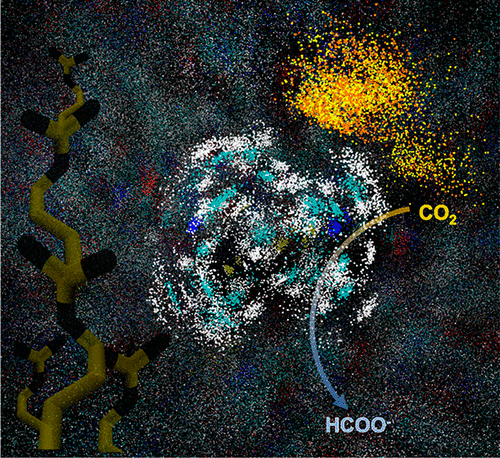
|
The protein scaffold around the active site of enzymes is known to influence catalytic activity, but specific scaffold features responsible for favorable influences are often not known. This study focuses on using an artificial metalloenzyme to probe one specific feature of the scaffold, the position of a positive charge in the outer coordination sphere around the active site. Previous work showed that a small molecular complex, [Rh(PEt2NglycinePEt2)2]−, immobilized covalently within a protein scaffold was activated for CO2 hydrogenation. Here, using an iterative design where the effect of arginine, histidine, or lysine residues placed in the outer coordination sphere of the catalytic active site were evaluated, we tested the hypothesis that positively charged groups facilitate CO2 hydrogenation with seven unique constructs. Single-, double-, and triple-point mutations were introduced to directly compare catalytic activity, as monitored by turnover frequencies (TOFs) measured in real time with 1H NMR spectroscopy, and evaluate related structural and electronic properties. Two of the seven constructs showed a 2- and 3-fold increase relative to the wild type, with overall rates ranging from 0.2 to 0.7 h–1, and a crystal structure of the fastest of these shows the positive charge positioned next to the active site. A crystal structure of the arginine-containing complex shows that the arginines are positioned near the metal. Molecular dynamics (MD) studies also suggest that the positive charge is oriented next to the active site in the two constructs with faster rates but not in the others and that the positive charge near the active site holds the CO2 near the metal, all consistent with a positive charge appropriately positioned in the scaffold benefiting catalysis. The MD studies also suggest that changes in the water distribution around the active site may contribute to catalytic activity, while modest structural changes and movement of the complex within the scaffold do not.
中文翻译:

人工酶的外部配位域中的正电荷会增加CO 2加氢
已知酶活性位点周围的蛋白质支架会影响催化活性,但是导致有利影响的特定支架特征通常是未知的。这项研究的重点是使用人工金属酶来探测支架的一个特定特征,即活性位点周围外协调球中正电荷的位置。先前的工作表明,共价固定在蛋白质支架中的小分子复合物[Rh(P Et 2 N甘氨酸P Et 2)2 ] -被激活了CO 2。氢化。在这里,使用一种迭代设计,其中评估了放置在催化活性位点外部配位域中的精氨酸,组氨酸或赖氨酸残基的作用,我们测试了带有七个独特结构的带正电基团有助于CO 2加氢的假设。引入单点,双点和三点突变以直接比较催化活性,如通过使用1 H NMR光谱实时测量的周转频率(TOF)进行监测,并评估相关的结构和电子性质。七个构建物中的两个显示相对于野生型增加了2倍和3倍,总速率为0.2到0.7 h –1,其中最快的晶体结构显示正电荷位于活性位点旁边。含精氨酸的络合物的晶体结构表明,精氨酸位于金属附近。分子动力学(MD)研究还表明,在两个结构中,正电荷的取向靠近活性位点,但速率较快,而在其他结构中则不然,并且活性位置附近的正电荷使金属附近的CO 2保持一致带有适当位置的正电荷,有利于催化。MD研究还表明,活性位点周围水分布的变化可能有助于催化活性,而适度的结构变化和复合物在支架内的运动则没有。
更新日期:2020-03-10
中文翻译:

人工酶的外部配位域中的正电荷会增加CO 2加氢
已知酶活性位点周围的蛋白质支架会影响催化活性,但是导致有利影响的特定支架特征通常是未知的。这项研究的重点是使用人工金属酶来探测支架的一个特定特征,即活性位点周围外协调球中正电荷的位置。先前的工作表明,共价固定在蛋白质支架中的小分子复合物[Rh(P Et 2 N甘氨酸P Et 2)2 ] -被激活了CO 2。氢化。在这里,使用一种迭代设计,其中评估了放置在催化活性位点外部配位域中的精氨酸,组氨酸或赖氨酸残基的作用,我们测试了带有七个独特结构的带正电基团有助于CO 2加氢的假设。引入单点,双点和三点突变以直接比较催化活性,如通过使用1 H NMR光谱实时测量的周转频率(TOF)进行监测,并评估相关的结构和电子性质。七个构建物中的两个显示相对于野生型增加了2倍和3倍,总速率为0.2到0.7 h –1,其中最快的晶体结构显示正电荷位于活性位点旁边。含精氨酸的络合物的晶体结构表明,精氨酸位于金属附近。分子动力学(MD)研究还表明,在两个结构中,正电荷的取向靠近活性位点,但速率较快,而在其他结构中则不然,并且活性位置附近的正电荷使金属附近的CO 2保持一致带有适当位置的正电荷,有利于催化。MD研究还表明,活性位点周围水分布的变化可能有助于催化活性,而适度的结构变化和复合物在支架内的运动则没有。











































 京公网安备 11010802027423号
京公网安备 11010802027423号