当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accelerated Cu2O Reduction by Single Pt Atoms at the Metal-Oxide Interface
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-19 , DOI: 10.1021/acscatal.9b05270 Alex C. Schilling 1 , Kyle Groden 2 , Juan Pablo Simonovis 3 , Adrian Hunt 3 , Ryan T. Hannagan 1 , Volkan Çınar 1 , Jean-Sabin McEwen 2, 4, 5, 6, 7 , E. Charles H. Sykes 1 , Iradwikanari Waluyo 3
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-19 , DOI: 10.1021/acscatal.9b05270 Alex C. Schilling 1 , Kyle Groden 2 , Juan Pablo Simonovis 3 , Adrian Hunt 3 , Ryan T. Hannagan 1 , Volkan Çınar 1 , Jean-Sabin McEwen 2, 4, 5, 6, 7 , E. Charles H. Sykes 1 , Iradwikanari Waluyo 3
Affiliation
|
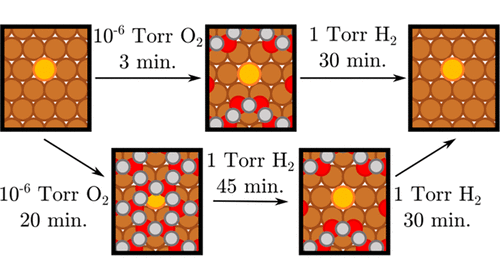
The reducibility of metal oxides, when they serve as the catalyst support or are the active sites themselves, plays an important role in heterogeneous catalytic reactions. Here we present an integrated experimental and theoretical study that reveals how the addition of small amounts of atomically dispersed Pt at the metal/oxide interface dramatically enhances the reducibility of a Cu2O thin film by H2. X-ray photoelectron spectroscopy (XPS) and temperature-programmed desorption (TPD) results reveal that, upon oxidation, a PtCu single-atom alloy (SAA) surface is covered by a thin Cu2O film and is, therefore, unable to dissociate H2. Despite this, in situ studies using ambient-pressure (AP) XPS reveal that the presence of a small amount of Pt under the oxide layer can, at the single-atom limit, promote the reduction of Cu2O by H2 at room temperature. We built two density functional theory based surface models to better understand these experimental findings: a Cu2O/Cu(111)-like surface oxide layer, known as the “29” oxide, in which Pt is alloyed into the Cu(111) surface, as well as a PtCu SAA. Our calculations suggest that the increased activity is due to the presence of atomically dispersed Pt under the surface oxide layer, which weakens the Cu–O bonds in its immediate vicinity, thus making the interface between subsurface Pt and the surface oxide a nucleation site for the formation of metallic Cu. This initial step in the reduction process results in the presence of surface Pt atoms surrounded by metallic Cu patches, and the Pt atoms become active in H2 dissociation, which consequently accelerates the reduction of the oxide layer. This work demonstrates how isolated Pt atoms at the metal/oxide interface of a Cu-based catalyst accelerate the reduction of the oxide and, therefore, help maintain the active, reduced state of the catalyst under the reaction conditions.
中文翻译:

金属氧化物界面上单个Pt原子加速的Cu 2 O还原
当金属氧化物用作催化剂载体或本身是活性位点时,其还原性在非均相催化反应中起重要作用。在这里,我们提出了一个综合的实验和理论研究,揭示了在金属/氧化物界面处添加少量原子分散的Pt如何显着提高H 2对Cu 2 O薄膜的还原性。X射线光电子能谱(XPS)和程序升温脱附(TPD)结果表明,氧化后,PtCu单原子合金(SAA)表面被薄的Cu 2 O薄膜覆盖,因此不能解离。 H 2。尽管如此,就地使用环境压力(AP)XPS进行的研究表明,在单原子限度下,氧化物层下少量Pt的存在可以促进室温下H 2对Cu 2 O的还原。我们建立了两个基于密度泛函理论的表面模型,以更好地理解这些实验结果:Cu 2O / Cu(111)状表面氧化物层(称为“ 29”氧化物)和PtCu SAA,其中Pt合金化到Cu(111)表面中。我们的计算表明,活性的增加是由于在表面氧化物层下存在原子分散的Pt,这削弱了其附近的Cu-O键,从而使表面下Pt和表面氧化物之间的界面成为Pt的成核位置。金属铜的形成。还原过程中的此初始步骤导致存在表面Pt原子被金属Cu斑包围,并且Pt原子在H 2中变得有活性离解,因此加速了氧化层的还原。这项工作证明了在铜基催化剂的金属/氧化物界面处孤立的Pt原子如何加速氧化物的还原,因此有助于在反应条件下保持催化剂的活性还原态。
更新日期:2020-03-20
中文翻译:

金属氧化物界面上单个Pt原子加速的Cu 2 O还原
当金属氧化物用作催化剂载体或本身是活性位点时,其还原性在非均相催化反应中起重要作用。在这里,我们提出了一个综合的实验和理论研究,揭示了在金属/氧化物界面处添加少量原子分散的Pt如何显着提高H 2对Cu 2 O薄膜的还原性。X射线光电子能谱(XPS)和程序升温脱附(TPD)结果表明,氧化后,PtCu单原子合金(SAA)表面被薄的Cu 2 O薄膜覆盖,因此不能解离。 H 2。尽管如此,就地使用环境压力(AP)XPS进行的研究表明,在单原子限度下,氧化物层下少量Pt的存在可以促进室温下H 2对Cu 2 O的还原。我们建立了两个基于密度泛函理论的表面模型,以更好地理解这些实验结果:Cu 2O / Cu(111)状表面氧化物层(称为“ 29”氧化物)和PtCu SAA,其中Pt合金化到Cu(111)表面中。我们的计算表明,活性的增加是由于在表面氧化物层下存在原子分散的Pt,这削弱了其附近的Cu-O键,从而使表面下Pt和表面氧化物之间的界面成为Pt的成核位置。金属铜的形成。还原过程中的此初始步骤导致存在表面Pt原子被金属Cu斑包围,并且Pt原子在H 2中变得有活性离解,因此加速了氧化层的还原。这项工作证明了在铜基催化剂的金属/氧化物界面处孤立的Pt原子如何加速氧化物的还原,因此有助于在反应条件下保持催化剂的活性还原态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号