当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering Caveolae-Targeted Lipid Nanoparticles To Deliver mRNA to the Lungs.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-03-13 , DOI: 10.1021/acschembio.0c00003 Qing Li , Chingshen Chan , Norman Peterson , Richard N Hanna , Alex Alfaro , Kevin L Allen , Herren Wu , William F Dall'Acqua , M Jack Borrok , Jose Luis Santos
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-03-13 , DOI: 10.1021/acschembio.0c00003 Qing Li , Chingshen Chan , Norman Peterson , Richard N Hanna , Alex Alfaro , Kevin L Allen , Herren Wu , William F Dall'Acqua , M Jack Borrok , Jose Luis Santos
|
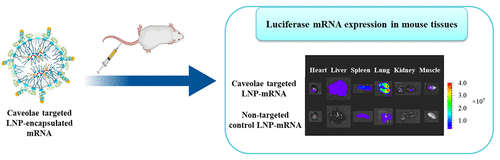
Efficacious use of therapeutic gene delivery via nanoparticles is hampered by the challenges associated with targeted delivery to tissues of interest. Systemic administration of lipid nanoparticle (LNP)-encapsulated mRNA leads to a protein expressed predominantly in the liver and spleen. Here, LNP encapsulating mRNA was covalently conjugated to an antibody, specifically binding plasmalemma vesicle-associated protein (PV1) as a means to target lung tissue. Systemic administration of PV1-targeted LNPs demonstrated significantly increased delivery of mRNA to the lungs and a 40-fold improvement in protein expression in the lungs, compared with control LNPs. We also investigated the effect of LNP size to determine optimal tissue distribution and transfection. Larger-size PV1-targeted LNPs not only have the elasticity to target the PV1 expressed in the caveolae but also enable robust mRNA expression in the lungs. Targeted delivery of mRNA to the lungs is a promising approach in the treatment of lung diseases.
中文翻译:

工程化针对Caveolae的脂质纳米颗粒,以将mRNA传递至肺。
经由纳米颗粒的治疗性基因递送的有效使用受到与靶向递送至感兴趣组织相关的挑战的阻碍。脂质纳米颗粒(LNP)封装的mRNA的全身给药会导致蛋白质主要在肝脏和脾脏中表达。在这里,将包裹LNP的mRNA与抗体共价缀合,特异性结合质膜囊泡相关蛋白(PV1)作为靶向肺组织的手段。与对照LNPs相比,以PV1为靶标的LNP的全身给药显示出向肺部的mRNA递送显着增加,并且肺部蛋白表达提高了40倍。我们还研究了LNP大小的影响,以确定最佳的组织分布和转染。较大尺寸的靶向PV1的LNP不仅具有弹性,可以靶向在小窝中表达的PV1,而且还可以使肺中的mRNA稳定表达。将mRNA靶向递送至肺部是治疗肺部疾病的有前途的方法。
更新日期:2020-04-23
中文翻译:

工程化针对Caveolae的脂质纳米颗粒,以将mRNA传递至肺。
经由纳米颗粒的治疗性基因递送的有效使用受到与靶向递送至感兴趣组织相关的挑战的阻碍。脂质纳米颗粒(LNP)封装的mRNA的全身给药会导致蛋白质主要在肝脏和脾脏中表达。在这里,将包裹LNP的mRNA与抗体共价缀合,特异性结合质膜囊泡相关蛋白(PV1)作为靶向肺组织的手段。与对照LNPs相比,以PV1为靶标的LNP的全身给药显示出向肺部的mRNA递送显着增加,并且肺部蛋白表达提高了40倍。我们还研究了LNP大小的影响,以确定最佳的组织分布和转染。较大尺寸的靶向PV1的LNP不仅具有弹性,可以靶向在小窝中表达的PV1,而且还可以使肺中的mRNA稳定表达。将mRNA靶向递送至肺部是治疗肺部疾病的有前途的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号