当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Covalent-Fragment Screening of BRD4 Identifies a Ligandable Site Orthogonal to the Acetyl-Lysine Binding Sites.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-23 , DOI: 10.1021/acschembio.0c00058 Michael D Olp 1 , Daniel J Sprague 1 , Christopher J Goetz 1 , Stefan G Kathman 2 , Sarah L Wynia-Smith 1 , Shifali Shishodia 1 , Steven B Summers 1 , Ziyang Xu 2 , Alexander V Statsyuk 2, 3 , Brian C Smith 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-23 , DOI: 10.1021/acschembio.0c00058 Michael D Olp 1 , Daniel J Sprague 1 , Christopher J Goetz 1 , Stefan G Kathman 2 , Sarah L Wynia-Smith 1 , Shifali Shishodia 1 , Steven B Summers 1 , Ziyang Xu 2 , Alexander V Statsyuk 2, 3 , Brian C Smith 1
Affiliation
|
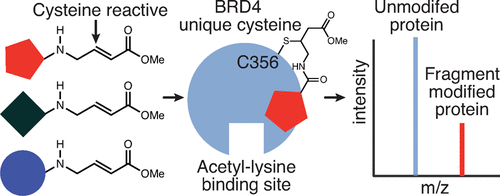
BRD4, a member of the bromodomain and extraterminal domain (BET) family, has emerged as a promising epigenetic target in cancer and inflammatory disorders. All reported BET family ligands bind within the bromodomain acetyl-lysine binding sites and competitively inhibit BET protein interaction with acetylated chromatin. Alternative chemical probes that act orthogonally to the highly conserved acetyl-lysine binding sites may exhibit selectivity within the BET family and avoid recently reported toxicity in clinical trials of BET bromodomain inhibitors. Here, we report the first identification of a ligandable site on a bromodomain outside the acetyl-lysine binding site. Inspired by our computational prediction of hotspots adjacent to nonhomologous cysteine residues within the C-terminal BRD4 bromodomain (BRD4-BD2), we performed a midthroughput mass spectrometry screen to identify cysteine-reactive fragments that covalently and selectively modify BRD4. Subsequent mass spectrometry, NMR, and computational docking analyses of electrophilic fragment hits revealed a novel ligandable site near Cys356 that is unique to BRD4 among human bromodomains. This site is orthogonal to the BRD4-BD2 acetyl-lysine binding site as Cys356 modification did not impact binding of the pan-BET bromodomain inhibitor JQ1 in fluorescence polarization assays nor an acetylated histone peptide in AlphaScreen assays. Finally, we tethered our top-performing covalent fragment to JQ1 and performed NanoBRET assays to provide proof of principle that this orthogonal site can be covalently targeted in intact human cells. Overall, we demonstrate the potential of targeting sites orthogonal to bromodomain acetyl-lysine binding sites to develop bivalent and covalent inhibitors that displace BRD4 from chromatin.
中文翻译:

BRD4 的共价片段筛选确定了与乙酰基-赖氨酸结合位点正交的可配位点。
BRD4 是溴结构域和末端外结构域 (BET) 家族的成员,已成为癌症和炎症性疾病中很有前景的表观遗传靶标。所有报道的 BET 家族配体均结合在溴结构域乙酰赖氨酸结合位点内,并竞争性抑制 BET 蛋白与乙酰化染色质的相互作用。与高度保守的乙酰赖氨酸结合位点正交作用的替代化学探针可能在 BET 家族中表现出选择性,并避免最近报道的 BET 溴结构域抑制剂临床试验中的毒性。在这里,我们报告了在乙酰赖氨酸结合位点之外的溴结构域上首次鉴定出可配位位点。受到我们对 C 端 BRD4 溴结构域 (BRD4-BD2) 内非同源半胱氨酸残基相邻热点的计算预测的启发,我们进行了中通量质谱筛选,以鉴定共价和选择性修饰 BRD4 的半胱氨酸反应片段。随后的质谱、NMR 和亲电子片段命中的计算对接分析揭示了 Cys356 附近的一个新的可配位点,该位点是人类溴结构域中 BRD4 所独有的。该位点与 BRD4-BD2 乙酰赖氨酸结合位点正交,因为 Cys356 修饰不影响荧光偏振测定中泛 BET 溴结构域抑制剂 JQ1 的结合,也不影响 AlphaScreen 测定中乙酰化组蛋白肽的结合。最后,我们将性能最佳的共价片段连接到 JQ1 上,并进行 NanoBRET 测定,以提供原理证明,证明该正交位点可以共价靶向完整的人类细胞。全面的,
更新日期:2020-04-23
中文翻译:

BRD4 的共价片段筛选确定了与乙酰基-赖氨酸结合位点正交的可配位点。
BRD4 是溴结构域和末端外结构域 (BET) 家族的成员,已成为癌症和炎症性疾病中很有前景的表观遗传靶标。所有报道的 BET 家族配体均结合在溴结构域乙酰赖氨酸结合位点内,并竞争性抑制 BET 蛋白与乙酰化染色质的相互作用。与高度保守的乙酰赖氨酸结合位点正交作用的替代化学探针可能在 BET 家族中表现出选择性,并避免最近报道的 BET 溴结构域抑制剂临床试验中的毒性。在这里,我们报告了在乙酰赖氨酸结合位点之外的溴结构域上首次鉴定出可配位位点。受到我们对 C 端 BRD4 溴结构域 (BRD4-BD2) 内非同源半胱氨酸残基相邻热点的计算预测的启发,我们进行了中通量质谱筛选,以鉴定共价和选择性修饰 BRD4 的半胱氨酸反应片段。随后的质谱、NMR 和亲电子片段命中的计算对接分析揭示了 Cys356 附近的一个新的可配位点,该位点是人类溴结构域中 BRD4 所独有的。该位点与 BRD4-BD2 乙酰赖氨酸结合位点正交,因为 Cys356 修饰不影响荧光偏振测定中泛 BET 溴结构域抑制剂 JQ1 的结合,也不影响 AlphaScreen 测定中乙酰化组蛋白肽的结合。最后,我们将性能最佳的共价片段连接到 JQ1 上,并进行 NanoBRET 测定,以提供原理证明,证明该正交位点可以共价靶向完整的人类细胞。全面的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号