当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Weak Intrinsic Luminescence in Monomeric Proteins Arising from Charge Recombination
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.jpcb.9b10071 Amrendra Kumar 1 , Dileep Ahari 1 , Anurag Priyadarshi 1 , Mohd. Ziauddin Ansari 1 , Rajaram Swaminathan 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.jpcb.9b10071 Amrendra Kumar 1 , Dileep Ahari 1 , Anurag Priyadarshi 1 , Mohd. Ziauddin Ansari 1 , Rajaram Swaminathan 1
Affiliation
|
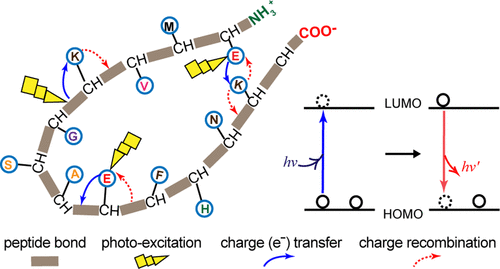
We had earlier reported on the presence of broad UV–vis electronic absorption (250–800 nm) in a monomeric protein rich in charged but lacking aromatic amino acids, referred to as Protein Charge Transfer Spectra (ProCharTS). Specifically, it was shown that the cationic amino/anionic carboxylate head groups of Lys/Glu side chains act as electronic charge acceptors/donors for photoinduced electron transfer either from/to the polypeptide backbone or to each other. In this work, we show that such excitations produce weak intrinsic luminescence in proteins originating from charge recombination. We investigated aqueous solutions of proteins with varying abundance of charged amino acids, like human serum albumin (HuSA) and hen lysozyme, and intrinsically disordered proteins, like PEST fragment of human c-Myc protein, α-synuclein, and dehydrin. The absorbance and luminescence in all protein samples were a linear function of the concentration (0–50 μM) employed, confirming their origin from a monomeric species. The slope of the luminescence/[protein] plot directly correlated with the fraction of charged amino acids present in protein. Specifically, the higher slope in proteins like HuSA was chiefly accounted by a large molar extinction coefficient rather than quantum yield. This coefficient directly correlates with the population of charged side-chain head groups lying in close spatial proximity in the protein, contributed by the three-dimensional (3D) fold of the polypeptide. ProCharTS luminescence parameters appear conserved across proteins. These include overlapping excitation/emission spectra, large Stokes shifts (14 000–3000 cm–1) that decrease with increasing excitation wavelength, low quantum yields (0.002–0.026) indicating poor radiative recombination efficiency, and multiexponential decays (mean lifetimes = 0.4–2.9 ns).
中文翻译:

电荷重组引起的单体蛋白内在发光弱。
我们之前曾报道过,在富含电荷但缺乏芳香族氨基酸的单体蛋白质中,存在广泛的紫外可见电子吸收(250–800 nm),称为蛋白质电荷转移谱(ProCharTS)。具体地,显示Lys / Glu侧链的阳离子氨基/阴离子羧酸根头基充当电荷诱导剂/供体,以用于从/至多肽主链或彼此之间的光致电子转移。在这项工作中,我们表明这种激发在源自电荷重组的蛋白质中产生弱的固有发光。我们研究了带电荷氨基酸含量丰富的蛋白质(例如人血清白蛋白(HuSA)和鸡溶菌酶)以及内在无序的蛋白质(例如人c-Myc蛋白的PEST片段,α-突触核蛋白和脱水素)的水溶液。所有蛋白质样品的吸光度和发光度都是所用浓度(0–50μM)的线性函数,证实了它们来自单体物种。发光/ [蛋白质]图的斜率与蛋白质中带电氨基酸的比例直接相关。具体来说,像HuSA这样的蛋白质中较高的斜率主要是由于摩尔消光系数大而不是量子产率所致。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 发光/ [蛋白质]图的斜率与蛋白质中带电氨基酸的比例直接相关。具体而言,像HuSA这样的蛋白质中较高的斜率主要是由较大的摩尔消光系数而不是量子产率引起的。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 发光/ [蛋白质]图的斜率与蛋白质中带电氨基酸的比例直接相关。具体而言,像HuSA这样的蛋白质中较高的斜率主要是由较大的摩尔消光系数而不是量子产率引起的。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 像HuSA这样的蛋白质中较高的斜率主要是由于摩尔消光系数大而不是量子产率所致。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 像HuSA这样的蛋白质中较高的斜率主要是由于摩尔消光系数大而不是量子产率所致。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm–1)随激发波长的增加而降低,量子产率低(0.002-0.026),表明辐射重组效率低,并且多指数衰减(平均寿命= 0.4-2.9 ns)。
更新日期:2020-03-26
中文翻译:

电荷重组引起的单体蛋白内在发光弱。
我们之前曾报道过,在富含电荷但缺乏芳香族氨基酸的单体蛋白质中,存在广泛的紫外可见电子吸收(250–800 nm),称为蛋白质电荷转移谱(ProCharTS)。具体地,显示Lys / Glu侧链的阳离子氨基/阴离子羧酸根头基充当电荷诱导剂/供体,以用于从/至多肽主链或彼此之间的光致电子转移。在这项工作中,我们表明这种激发在源自电荷重组的蛋白质中产生弱的固有发光。我们研究了带电荷氨基酸含量丰富的蛋白质(例如人血清白蛋白(HuSA)和鸡溶菌酶)以及内在无序的蛋白质(例如人c-Myc蛋白的PEST片段,α-突触核蛋白和脱水素)的水溶液。所有蛋白质样品的吸光度和发光度都是所用浓度(0–50μM)的线性函数,证实了它们来自单体物种。发光/ [蛋白质]图的斜率与蛋白质中带电氨基酸的比例直接相关。具体来说,像HuSA这样的蛋白质中较高的斜率主要是由于摩尔消光系数大而不是量子产率所致。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 发光/ [蛋白质]图的斜率与蛋白质中带电氨基酸的比例直接相关。具体而言,像HuSA这样的蛋白质中较高的斜率主要是由较大的摩尔消光系数而不是量子产率引起的。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 发光/ [蛋白质]图的斜率与蛋白质中带电氨基酸的比例直接相关。具体而言,像HuSA这样的蛋白质中较高的斜率主要是由较大的摩尔消光系数而不是量子产率引起的。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 像HuSA这样的蛋白质中较高的斜率主要是由于摩尔消光系数大而不是量子产率所致。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm 像HuSA这样的蛋白质中较高的斜率主要是由于摩尔消光系数大而不是量子产率所致。该系数与蛋白质中空间紧密接近的带电侧链头基团的数量直接相关,这是由多肽的三维(3D)折叠引起的。ProCharTS发光参数在整个蛋白质中看起来是保守的。这些包括重叠的激发/发射光谱,较大的斯托克斯位移(14 000–3000 cm–1)随激发波长的增加而降低,量子产率低(0.002-0.026),表明辐射重组效率低,并且多指数衰减(平均寿命= 0.4-2.9 ns)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号