当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamics of Water in the Solvation Shell of an Iodate Ion: A Born–Oppenheimer Molecular Dynamics Study
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpcb.9b12008 Bikramjit Sharma 1 , Amalendu Chandra 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpcb.9b12008 Bikramjit Sharma 1 , Amalendu Chandra 1
Affiliation
|
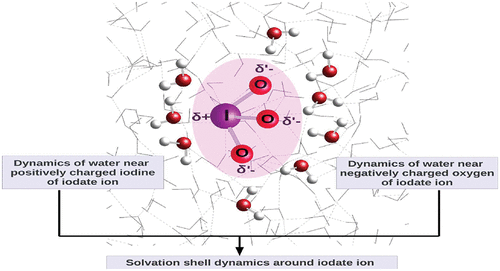
The iodate ion has an anisotropic structure and charge distribution. It has a pyramidal shape with the iodine atom located at the peak of the pyramid. The water molecules interact differently with the positively charged iodine and the negatively charged oxygen atoms of this anion, giving rise to two distinct solvation shells. In the present study, we have performed ab initio Born–Oppenheimer molecular dynamics simulations to investigate the dynamics of water molecules in the iodine and oxygen solvation shells of the iodate ion and compared the behavior with those of the bulk. The dynamics of water is calculated for both the BLYP and the dispersion-corrected BLYP-D3 functionals at room temperature. The dynamics of water in the solvation shells at higher temperatures of 353 and 330 K has also been investigated for the BLYP and BLYP-D3 functionals, respectively. The hydrogen bond dynamics, vibrational spectral diffusion, orientational and translational diffusion, and residence dynamics of water molecules in the two solvation shells are looked at in the current study. The ion–water hydrogen bond dynamics is found to be somewhat faster than that for water–water hydrogen bonds in the bulk, which can be attributed to a ring-like electron distribution on the iodate oxygens. The dynamical trends are connected to the water structure making/breaking properties of the positively charged iodine and negatively charged oxygen sites of the anion. Furthermore, orientational jumps of the iodate ion and also those of surrounding water molecules which are hydrogen bonded to the oxygen atoms of the iodate ion are also investigated. It is found that the nature of these orientational jumps can be different from those reported earlier for planar polyoxyanions such as the nitrate ion.
中文翻译:

碘离子溶剂壳中水的动力学:Born–Oppenheimer分子动力学研究
碘酸根离子具有各向异性的结构和电荷分布。它具有金字塔形状,碘原子位于金字塔的顶点。水分子与该阴离子的带正电的碘和带负电的氧原子发生不同的相互作用,产生两个不同的溶剂化壳。在本研究中,我们从头进行了Born–Oppenheimer分子动力学模拟,以研究碘酸根离子在碘和氧的溶剂化壳中水分子的动力学并将其行为与本体的行为进行比较。计算室温下BLYP和经分散校正的BLYP-D3官能团的水动力学。还针对BLYP和BLYP-D3官能团研究了在353和330 K较高温度下溶剂化壳中水的动力学,分别。在当前研究中,研究了氢键动力学,振动光谱扩散,取向和平移扩散以及两个溶剂化壳中水分子的驻留动力学。发现离子-水氢键动力学比整体中的水-水氢键动力学快一些,这可以归因于碘酸盐氧上的环状电子分布。动态趋势与阴离子的带正电的碘和带负电的氧位的水结构产生/破坏特性有关。此外,还研究了碘酸根离子的取向跃迁以及氢键合到碘酸根离子的氧原子上的周围水分子的取向跃迁。
更新日期:2020-03-21
中文翻译:

碘离子溶剂壳中水的动力学:Born–Oppenheimer分子动力学研究
碘酸根离子具有各向异性的结构和电荷分布。它具有金字塔形状,碘原子位于金字塔的顶点。水分子与该阴离子的带正电的碘和带负电的氧原子发生不同的相互作用,产生两个不同的溶剂化壳。在本研究中,我们从头进行了Born–Oppenheimer分子动力学模拟,以研究碘酸根离子在碘和氧的溶剂化壳中水分子的动力学并将其行为与本体的行为进行比较。计算室温下BLYP和经分散校正的BLYP-D3官能团的水动力学。还针对BLYP和BLYP-D3官能团研究了在353和330 K较高温度下溶剂化壳中水的动力学,分别。在当前研究中,研究了氢键动力学,振动光谱扩散,取向和平移扩散以及两个溶剂化壳中水分子的驻留动力学。发现离子-水氢键动力学比整体中的水-水氢键动力学快一些,这可以归因于碘酸盐氧上的环状电子分布。动态趋势与阴离子的带正电的碘和带负电的氧位的水结构产生/破坏特性有关。此外,还研究了碘酸根离子的取向跃迁以及氢键合到碘酸根离子的氧原子上的周围水分子的取向跃迁。











































 京公网安备 11010802027423号
京公网安备 11010802027423号