当前位置:
X-MOL 学术
›
Adv. Opt. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote Modification of Bidentate Phosphane Ligands Controlling the Photonic Properties in Their Complexes: Enhanced Performance of [Cu(RN‐xantphos)(N^N)][PF6] in Light‐Emitting Electrochemical Cells
Advanced Optical Materials ( IF 8.0 ) Pub Date : 2020-03-09 , DOI: 10.1002/adom.201901689 Nina Arnosti 1 , Fabian Brunner 1 , Isidora Susic 2 , Sarah Keller 1 , José M. Junquera‐Hernández 2 , Alessandro Prescimone 1 , Henk J. Bolink 2 , Michele Sessolo 2 , Enrique Ortí 2 , Catherine E. Housecroft 1 , Edwin C. Constable 1
Advanced Optical Materials ( IF 8.0 ) Pub Date : 2020-03-09 , DOI: 10.1002/adom.201901689 Nina Arnosti 1 , Fabian Brunner 1 , Isidora Susic 2 , Sarah Keller 1 , José M. Junquera‐Hernández 2 , Alessandro Prescimone 1 , Henk J. Bolink 2 , Michele Sessolo 2 , Enrique Ortí 2 , Catherine E. Housecroft 1 , Edwin C. Constable 1
Affiliation
|
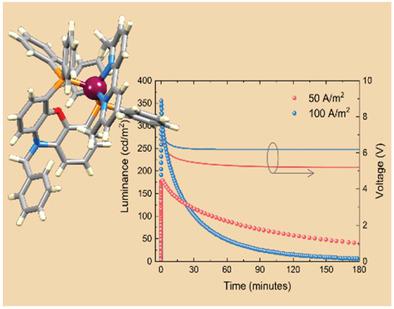
A series of copper(I) complexes of the type [Cu(HN‐xantphos)(N^N)][PF6] and [Cu(BnN‐xantphos)(N^N)][PF6], in which N^N = bpy, Mebpy, and Me2bpy, HN‐xantphos = 4,6‐bis(diphenylphosphanyl)‐10H‐phenoxazine and BnN‐xantphos = 10‐benzyl‐4,6‐bis(diphenylphosphanyl)‐10H‐phenoxazine is described. The single crystal structures of [Cu(HN‐xantphos)(Mebpy)][PF6] and [Cu(BnN‐xantphos)(Me2bpy)][PF6] confirm the presence of N^N and P^P chelating ligands with the copper(I) atoms in distorted coordination environments. Solution electrochemical and photophysical properties of the BnN‐xantphos‐containing compounds (for which the highest‐occupied molecular orbital is located on the phenoxazine moiety) are reported. The first oxidation of [Cu(BnN‐xantphos)(N^N)][PF6] occurs on the BnN‐xantphos ligand. Time‐dependent density functional theory (TD‐DFT) calculations have been used to analyze the solution absorption spectra of the [Cu(BnN‐xantphos)(N^N)][PF6] compounds. In the solid‐state, the compounds show photoluminescence in the range 518–555 nm for [Cu(HN‐xantphos)(N^N)][PF6] and 520–575 nm for [Cu(BnN‐xantphos)(N^N)][PF6] with a blue‐shift on going from bpy to Mebpy to Me2bpy. [Cu(BnN‐xantphos)(Me2bpy)][PF6] exhibits a solid‐state photoluminescence quantum yield of 55% with an excited state lifetime of 17.4 µs. Bright light‐emitting electrochemical cells are obtained using this complex, and it is shown that the electroluminescence quantum yield can be enhanced by using less conducting hole injection layers.
中文翻译:

双峰磷配体的远程修饰,控制其配合物的光子性质:[Cu(RN-xantphos)(N ^ N)] [PF6]在发光电化学电池中的增强性能
[[Cu(HN-xantphos)(N ^ N)] [PF 6 ]和[Cu(BnN-xantphos)(N ^ N)] [PF 6 ]类型的一系列铜(I)配合物,其中N ^ N = bpy,Mebpy和Me 2 bpy,HN-xantphos = 4,6-双(二苯基膦基)-10H-吩恶嗪和BnN-xantphos = 10-苄基-4,6-双(二苯基膦基)-10H-吩恶嗪为描述。[Cu(HN-xantphos)(Mebpy)] [PF 6 ]和[Cu(BnN-xantphos)(Me 2 bpy)] [PF 6 ]的单晶结构证实了N ^ N和P ^的存在在扭曲的配位环境中与铜(I)原子形成P螯合配体。据报道,含BnN-黄药的化合物的溶液电化学和光物理性质(占据最多的分子轨道位于吩恶嗪部分上)。[Cu(BnN-xantphos)(N ^ N)] [PF 6 ]的第一次氧化发生在BnN-xantphos配体上。随时间变化的密度泛函理论(TD-DFT)计算已用于分析[Cu(BnN-xantphos)(N ^ N)] [PF 6 ]化合物的溶液吸收光谱。在固态中,化合物对[Cu(HN-xantphos)(N ^ N)] [PF 6 ]的发光范围为518-555 nm,对于[Cu(BnN-xantphos)(N^ N)] [PF 6 ],从bpy到Mebpy再到Me 2 bpy发生了蓝移。[Cu(BnN-xantphos)(Me 2 bpy)] [PF 6 ]的固态光致发光量子产率为55%,激发态寿命为17.4 µs。使用该配合物可以获得明亮的发光电化学电池,并且表明通过使用较少的导电空穴注入层可以提高电致发光量子产率。
更新日期:2020-03-09
中文翻译:

双峰磷配体的远程修饰,控制其配合物的光子性质:[Cu(RN-xantphos)(N ^ N)] [PF6]在发光电化学电池中的增强性能
[[Cu(HN-xantphos)(N ^ N)] [PF 6 ]和[Cu(BnN-xantphos)(N ^ N)] [PF 6 ]类型的一系列铜(I)配合物,其中N ^ N = bpy,Mebpy和Me 2 bpy,HN-xantphos = 4,6-双(二苯基膦基)-10H-吩恶嗪和BnN-xantphos = 10-苄基-4,6-双(二苯基膦基)-10H-吩恶嗪为描述。[Cu(HN-xantphos)(Mebpy)] [PF 6 ]和[Cu(BnN-xantphos)(Me 2 bpy)] [PF 6 ]的单晶结构证实了N ^ N和P ^的存在在扭曲的配位环境中与铜(I)原子形成P螯合配体。据报道,含BnN-黄药的化合物的溶液电化学和光物理性质(占据最多的分子轨道位于吩恶嗪部分上)。[Cu(BnN-xantphos)(N ^ N)] [PF 6 ]的第一次氧化发生在BnN-xantphos配体上。随时间变化的密度泛函理论(TD-DFT)计算已用于分析[Cu(BnN-xantphos)(N ^ N)] [PF 6 ]化合物的溶液吸收光谱。在固态中,化合物对[Cu(HN-xantphos)(N ^ N)] [PF 6 ]的发光范围为518-555 nm,对于[Cu(BnN-xantphos)(N^ N)] [PF 6 ],从bpy到Mebpy再到Me 2 bpy发生了蓝移。[Cu(BnN-xantphos)(Me 2 bpy)] [PF 6 ]的固态光致发光量子产率为55%,激发态寿命为17.4 µs。使用该配合物可以获得明亮的发光电化学电池,并且表明通过使用较少的导电空穴注入层可以提高电致发光量子产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号