当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microwave‐assisted synthesis and luminescent activity of imidazo[1,2‐a]pyridine derivatives
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-07 , DOI: 10.1002/jhet.3950 Juan C. Rodríguez 1 , Rony A. Maldonado 1 , Gonzalo Ramírez‐García 2 , Erik Díaz Cervantes 3 , Fabiola N. Cruz 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-07 , DOI: 10.1002/jhet.3950 Juan C. Rodríguez 1 , Rony A. Maldonado 1 , Gonzalo Ramírez‐García 2 , Erik Díaz Cervantes 3 , Fabiola N. Cruz 1
Affiliation
|
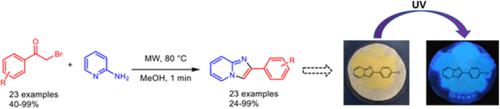
In this work, a series of phenacyl bromide derivatives was synthesized and employed as key intermediate for the synthesis of substituted imidazo[1,2‐a]pyridines. First, phenacyl bromide molecules were obtained from the bromination reaction of acetophenones assisted by microwave irradiation, obtaining the products 4a‐v in a 15 minutes reaction with yields in the range of 50% to 99%. Subsequently, the conjugation of these molecules with 2‐aminopyridine conduced to the production of imidazo[1,2‐a]pyridine derivatives (7a‐v) in a 60‐second reaction with yields of 24% to 99%. Improved yields were determined with respect to those obtained with more tedious methodologies like thermally and mechanically assisted routes. Intense luminescence emissions in the purple and blue regions of the electromagnetic spectra were observed under UV excitation according to the nature of the substituents. This environmentally friendly methodology is expected to constitute an important class of organic compounds for the development of biomarkers, photochemical sensors, and medicinal applications.
中文翻译:

咪唑并[1,2-a]吡啶衍生物的微波辅助合成和发光活性
在这项工作中,合成了一系列苯甲酰溴衍生物,并用作合成取代的咪唑并[1,2- a ]吡啶的关键中间体。首先,在微波辐射的辅助下,通过苯乙酮的溴化反应获得苯甲酰溴分子,在15分钟的反应中获得产物4a-v,产率在50%至99%之间。随后,这些分子与2-氨基吡啶共轭,产生了咪唑并[1,2- a ]吡啶衍生物(7a-v)在60秒的反应中产率为24%至99%。相对于通过更繁琐的方法(如热辅助和机械辅助路线)获得的产量,确定了更高的产量。根据取代基的性质,在紫外线激发下观察到电磁光谱的紫色和蓝色区域中的强烈发光发射。这种对环境友好的方法有望构成一类重要的有机化合物,用于开发生物标志物,光化学传感器和医学应用。
更新日期:2020-04-22
中文翻译:

咪唑并[1,2-a]吡啶衍生物的微波辅助合成和发光活性
在这项工作中,合成了一系列苯甲酰溴衍生物,并用作合成取代的咪唑并[1,2- a ]吡啶的关键中间体。首先,在微波辐射的辅助下,通过苯乙酮的溴化反应获得苯甲酰溴分子,在15分钟的反应中获得产物4a-v,产率在50%至99%之间。随后,这些分子与2-氨基吡啶共轭,产生了咪唑并[1,2- a ]吡啶衍生物(7a-v)在60秒的反应中产率为24%至99%。相对于通过更繁琐的方法(如热辅助和机械辅助路线)获得的产量,确定了更高的产量。根据取代基的性质,在紫外线激发下观察到电磁光谱的紫色和蓝色区域中的强烈发光发射。这种对环境友好的方法有望构成一类重要的有机化合物,用于开发生物标志物,光化学传感器和医学应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号