当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Food Amyloid Protein Digestion by Conical Nanopore
Small Methods ( IF 10.7 ) Pub Date : 2020-03-08 , DOI: 10.1002/smtd.201900703 Nicoletta Giamblanco 1 , Jean‐Marc Janot 1 , Alberto Gubbiotti 2 , Mauro Chinappi 3 , Sebastien Balme 1
Small Methods ( IF 10.7 ) Pub Date : 2020-03-08 , DOI: 10.1002/smtd.201900703 Nicoletta Giamblanco 1 , Jean‐Marc Janot 1 , Alberto Gubbiotti 2 , Mauro Chinappi 3 , Sebastien Balme 1
Affiliation
|
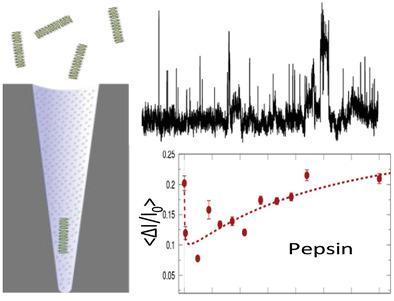
β‐lactoglobulin aggregates are used as structuring agents in food processing. Their enzymatic degradation follows different pathways depending on the enzyme and the pH. The transient species produced during the degradation process are difficult to characterize under continuous measurement. Here, conical track‐etched nanopores are used to investigate the β‐lactoglobulin degradation by pepsin (pH 2) and trypsin (pH 9). Before enzyme addition, two distinct populations, oligomers and protofibrils, are identified. Just after enzyme addition, the aggregate size decreases. For pepsin, a phenomenon of reaggregation that does not occur for the trypsin is evidenced. In addition, after 140 min a larger population of aggregate appears. The experimental results are supported by a kinetic model. This work also demonstrates that conical nanopore allows following the kinetic of transient protein aggregate intermediates during enzymatic degradation.
中文翻译:

锥形纳米孔对食物淀粉样蛋白消化的表征
β-乳球蛋白聚集体在食品加工中用作结构化剂。它们的酶促降解遵循不同的途径,具体取决于酶和pH。在降解过程中产生的瞬态物质很难在连续测量下表征。在这里,圆锥形轨迹蚀刻纳米孔用于研究胃蛋白酶(pH 2)和胰蛋白酶(pH 9)对β-乳球蛋白的降解作用。在添加酶之前,确定了两个不同的群体,即低聚物和原纤维。刚添加酶后,聚集体尺寸减小。对于胃蛋白酶,胰蛋白酶不会发生重新聚集现象。另外,在140分钟后,出现了更大的聚集体。实验结果得到动力学模型的支持。
更新日期:2020-03-08
中文翻译:

锥形纳米孔对食物淀粉样蛋白消化的表征
β-乳球蛋白聚集体在食品加工中用作结构化剂。它们的酶促降解遵循不同的途径,具体取决于酶和pH。在降解过程中产生的瞬态物质很难在连续测量下表征。在这里,圆锥形轨迹蚀刻纳米孔用于研究胃蛋白酶(pH 2)和胰蛋白酶(pH 9)对β-乳球蛋白的降解作用。在添加酶之前,确定了两个不同的群体,即低聚物和原纤维。刚添加酶后,聚集体尺寸减小。对于胃蛋白酶,胰蛋白酶不会发生重新聚集现象。另外,在140分钟后,出现了更大的聚集体。实验结果得到动力学模型的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号