Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NAG‐thiazoline is a potent inhibitor of the Vibrio campbellii GH20 β‐N‐Acetylglucosaminidase
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-07 , DOI: 10.1111/febs.15283 Piyanat Meekrathok 1 , Keith A. Stubbs 2 , Anuwat Aunkham 3 , Anuphon Kaewmaneewat 3 , Apinya Kardkuntod 3 , David M Bulmer 4 , Bert van den Berg 4 , Wipa Suginta 3
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-07 , DOI: 10.1111/febs.15283 Piyanat Meekrathok 1 , Keith A. Stubbs 2 , Anuwat Aunkham 3 , Anuphon Kaewmaneewat 3 , Apinya Kardkuntod 3 , David M Bulmer 4 , Bert van den Berg 4 , Wipa Suginta 3
Affiliation
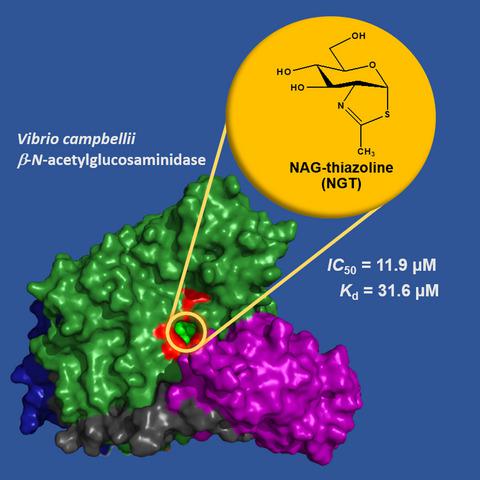
|
Vibrio spp. play a vital role in the recycling of chitin in oceans, but several Vibrio strains are highly infectious to aquatic animals and humans. These bacteria require chitin for growth; thus, potent inhibitors of chitin‐degrading enzymes could serve as candidate drugs against Vibrio infections. This study examined NAG‐thiazoline (NGT)‐mediated inhibition of a recombinantly expressed GH20 β‐N‐acetylglucosaminidase, namely VhGlcNAcase from Vibrio campbellii (formerly V. harveyi) ATCC BAA‐1116. NGT strongly inhibited VhGlcNAcase with an IC50 of 11.9 ± 1.0 μm and Ki 62 ± 3 µm, respectively. NGT was also found to completely inhibit the growth of V. campbellii strain 650 with an minimal inhibitory concentration value of 0.5 µm. ITC data analysis showed direct binding of NGT to VhGlcNAcase with a Kd of 32 ± 1.2 μm. The observed ΔG°binding of −7.56 kcal·mol−1 is the result of a large negative enthalpy change and a small positive entropic compensation, suggesting that NGT binding is enthalpy‐driven. The structural complex shows that NGT fully occupies the substrate‐binding pocket of VhGlcNAcase and makes an exclusive hydrogen bond network, as well as hydrophobic interactions with the conserved residues around the −1 subsite. Our results strongly suggest that NGT could serve as an excellent scaffold for further development of antimicrobial agents against Vibrio infections.
中文翻译:

NAG-噻唑啉是一种有效的坎氏弧菌GH20β-N-乙酰氨基葡萄糖苷酶抑制剂
弧菌属。几丁质弧菌在海洋中的几丁质循环中起着至关重要的作用,但它们对水生动物和人类具有高度传染性。这些细菌需要几丁质才能生长。因此,几丁质降解酶的有效抑制剂可以作为抗弧菌感染的候选药物。本研究NAG噻唑啉(NGT)介导的抑制的重组表达的GH20β- Ñ -acetylglucosaminidase,即VH GlcNAcase从坎氏弧菌(以前哈氏弧菌)ATCC BAA-1116。NGT强烈抑制VH GlcNAcase与IC 50的11.9±1.0μ米和ķ我62±3μ米,分别。NGT还发现完全抑制生长V.坎应变650,用0.5μ的最小抑制浓度值米。ITC数据分析表明直接到NGT的结合VH与GlcNAcase ķ d的32±1.2μ米。观察到的-7.56 kcal·mol -1的ΔG °结合是大的负焓变化和小的正熵补偿的结果,这表明NGT结合是由焓驱动的。结构复合物表明NGT完全占据了Vh的底物结合口袋GlcNAcase并形成一个排他的氢键网络,以及与-1亚位点周围保守残基的疏水相互作用。我们的结果强烈表明,NGT可以作为进一步开发抗弧菌感染的抗菌剂的优良支架。
更新日期:2020-03-07
中文翻译:

NAG-噻唑啉是一种有效的坎氏弧菌GH20β-N-乙酰氨基葡萄糖苷酶抑制剂
弧菌属。几丁质弧菌在海洋中的几丁质循环中起着至关重要的作用,但它们对水生动物和人类具有高度传染性。这些细菌需要几丁质才能生长。因此,几丁质降解酶的有效抑制剂可以作为抗弧菌感染的候选药物。本研究NAG噻唑啉(NGT)介导的抑制的重组表达的GH20β- Ñ -acetylglucosaminidase,即VH GlcNAcase从坎氏弧菌(以前哈氏弧菌)ATCC BAA-1116。NGT强烈抑制VH GlcNAcase与IC 50的11.9±1.0μ米和ķ我62±3μ米,分别。NGT还发现完全抑制生长V.坎应变650,用0.5μ的最小抑制浓度值米。ITC数据分析表明直接到NGT的结合VH与GlcNAcase ķ d的32±1.2μ米。观察到的-7.56 kcal·mol -1的ΔG °结合是大的负焓变化和小的正熵补偿的结果,这表明NGT结合是由焓驱动的。结构复合物表明NGT完全占据了Vh的底物结合口袋GlcNAcase并形成一个排他的氢键网络,以及与-1亚位点周围保守残基的疏水相互作用。我们的结果强烈表明,NGT可以作为进一步开发抗弧菌感染的抗菌剂的优良支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号