Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-content imaging and structure-based predictions reveal functional differences between Niemann-Pick C1 variants.
Traffic ( IF 3.6 ) Pub Date : 2020-04-05 , DOI: 10.1111/tra.12727 Lauri Vanharanta 1, 2 , Johan Peränen 1, 2 , Simon G Pfisterer 1 , Giray Enkavi 3, 4 , Ilpo Vattulainen 3, 4 , Elina Ikonen 1, 2
Traffic ( IF 3.6 ) Pub Date : 2020-04-05 , DOI: 10.1111/tra.12727 Lauri Vanharanta 1, 2 , Johan Peränen 1, 2 , Simon G Pfisterer 1 , Giray Enkavi 3, 4 , Ilpo Vattulainen 3, 4 , Elina Ikonen 1, 2
Affiliation
|
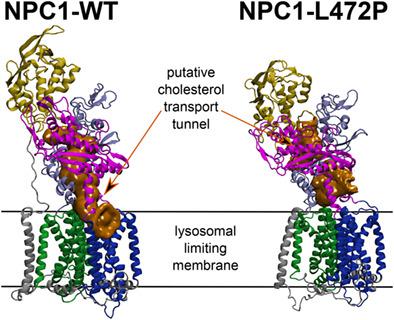
The human Niemann-Pick C1 (NPC1) gene encoding a 1278 amino acid protein is very heterogeneous. While some variants represent benign polymorphisms, NPC disease carriers and patients may possess rare variants, whose functional importance remains unknown. An NPC1 cDNA construct known as NPC1 wild-type variant (WT-V), distributed between laboratories and used as a WT control in several studies, also contains changes regarding specific amino acids compared to the NPC1 Genbank reference sequence. To improve the dissection of subtle functional differences, we generated human cells stably expressing NPC1 variants from the AAVS1 safe-harbor locus on an NPC1-null background engineered by CRISPR/Cas9 editing. We then employed high-content imaging with automated image analysis to quantitatively assess LDL-induced, time-dependent changes in lysosomal cholesterol content and lipid droplet formation. Our results indicate that the L472P change present in NPC1 WT-V compromises NPC1 functionality in lysosomal cholesterol export. All-atom molecular dynamics simulations suggest that the L472P change alters the relative position of the NPC1 middle and the C-terminal luminal domains, disrupting the recently characterized cholesterol efflux tunnel. These results reveal functional defects in NPC1 WT-V and highlight the strength of simulations and quantitative imaging upon stable protein expression in elucidating subtle differences in protein function.
中文翻译:

高内涵成像和基于结构的预测揭示了Niemann-Pick C1变体之间的功能差异。
编码1278个氨基酸蛋白质的人Niemann-Pick C1(NPC1)基因非常异质。尽管某些变体代表良性多态性,但NPC疾病携带者和患者可能拥有罕见的变体,其功能重要性仍然未知。NPC1 cDNA构建体称为NPC1野生型变体(WT-V),分布在实验室之间,在一些研究中用作WT对照。与NPC1 Genbank参考序列相比,NPC1 cDNA还包含有关特定氨基酸的变化。为了改善细微的功能差异的解剖,我们在由CRISPR / Cas9编辑设计的NPC1无背景上生成了从AAVS1安全港基因座稳定表达NPC1变体的人细胞。然后,我们采用高内涵成像和自动图像分析技术来定量评估LDL诱导的,溶酶体胆固醇含量和脂质滴形成的时间依赖性变化。我们的结果表明,存在于NPC1 WT-V中的L472P变化损害了溶酶体胆固醇输出中的NPC1功能。全原子分子动力学模拟表明,L472P的变化改变了NPC1中部和C末端腔结构域的相对位置,破坏了最近表征的胆固醇流出通道。这些结果揭示了NPC1 WT-V的功能缺陷,并突出了稳定蛋白表达下的模拟和定量成像的强度,以阐明蛋白功能的细微差别。全原子分子动力学模拟表明,L472P的变化改变了NPC1中部和C末端腔结构域的相对位置,破坏了最近表征的胆固醇流出通道。这些结果揭示了NPC1 WT-V的功能缺陷,并突出了稳定蛋白表达下的模拟和定量成像的强度,以阐明蛋白功能的细微差别。全原子分子动力学模拟表明,L472P的变化改变了NPC1中部和C末端腔结构域的相对位置,破坏了最近表征的胆固醇流出通道。这些结果揭示了NPC1 WT-V的功能缺陷,并突出了稳定蛋白表达下的模拟和定量成像的强度,以阐明蛋白功能的细微差别。
更新日期:2020-04-05
中文翻译:

高内涵成像和基于结构的预测揭示了Niemann-Pick C1变体之间的功能差异。
编码1278个氨基酸蛋白质的人Niemann-Pick C1(NPC1)基因非常异质。尽管某些变体代表良性多态性,但NPC疾病携带者和患者可能拥有罕见的变体,其功能重要性仍然未知。NPC1 cDNA构建体称为NPC1野生型变体(WT-V),分布在实验室之间,在一些研究中用作WT对照。与NPC1 Genbank参考序列相比,NPC1 cDNA还包含有关特定氨基酸的变化。为了改善细微的功能差异的解剖,我们在由CRISPR / Cas9编辑设计的NPC1无背景上生成了从AAVS1安全港基因座稳定表达NPC1变体的人细胞。然后,我们采用高内涵成像和自动图像分析技术来定量评估LDL诱导的,溶酶体胆固醇含量和脂质滴形成的时间依赖性变化。我们的结果表明,存在于NPC1 WT-V中的L472P变化损害了溶酶体胆固醇输出中的NPC1功能。全原子分子动力学模拟表明,L472P的变化改变了NPC1中部和C末端腔结构域的相对位置,破坏了最近表征的胆固醇流出通道。这些结果揭示了NPC1 WT-V的功能缺陷,并突出了稳定蛋白表达下的模拟和定量成像的强度,以阐明蛋白功能的细微差别。全原子分子动力学模拟表明,L472P的变化改变了NPC1中部和C末端腔结构域的相对位置,破坏了最近表征的胆固醇流出通道。这些结果揭示了NPC1 WT-V的功能缺陷,并突出了稳定蛋白表达下的模拟和定量成像的强度,以阐明蛋白功能的细微差别。全原子分子动力学模拟表明,L472P的变化改变了NPC1中部和C末端腔结构域的相对位置,破坏了最近表征的胆固醇流出通道。这些结果揭示了NPC1 WT-V的功能缺陷,并突出了稳定蛋白表达下的模拟和定量成像的强度,以阐明蛋白功能的细微差别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号