当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron‐Mediated Ring‐Opening and Rearrangement Cascade Synthesis of Polysubstituted Pyrroles from 4‐Alkenylisoxazoles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-30 , DOI: 10.1002/adsc.201901649 Wen Yang 1, 2 , Xiaochen Liu 1, 3 , Pak‐Hing Leung 4 , Yongxin Li 4 , Dingqiao Yang 2 , Yu Chen 1, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-30 , DOI: 10.1002/adsc.201901649 Wen Yang 1, 2 , Xiaochen Liu 1, 3 , Pak‐Hing Leung 4 , Yongxin Li 4 , Dingqiao Yang 2 , Yu Chen 1, 3
Affiliation
|
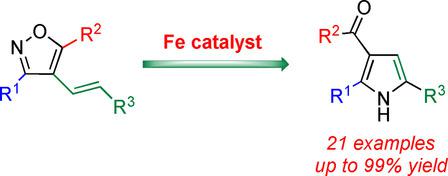
An iron‐mediated ring‐opening and rearrangement cascade from 4‐alkenylisoxazoles to polysubstituted pyrroles is described. The cascade reaction conditions are compatible with a variety of functional groups, including ketones, esters, amides, and aryl halides. High reactivity is maintained even if the reaction scale is expanded to 0.5 grams. It is the first iron‐catalyzed rearrangement of 4‐alkenylisoxazoles to pyrroles that is independent of the presence of the alkoxy or amino group at position 5 of the isoxazole substrates. A plausible mechanism of the iron‐mediated cascade reaction is proposed.
中文翻译:

铁介导的开环和重排级联反应,由4-烯基异恶唑合成多取代的吡咯
描述了铁介导的开环和重排从4-烯基异恶唑到多取代吡咯的级联反应。级联反应条件与多种官能团相容,包括酮,酯,酰胺和卤代芳基。即使反应规模扩大到0.5克,也保持了高反应性。这是第一个铁催化的4-烯基异恶唑向吡咯的重排,与异恶唑底物第5位上烷氧基或氨基的存在无关。提出了铁介导的级联反应的合理机制。
更新日期:2020-03-30
中文翻译:

铁介导的开环和重排级联反应,由4-烯基异恶唑合成多取代的吡咯
描述了铁介导的开环和重排从4-烯基异恶唑到多取代吡咯的级联反应。级联反应条件与多种官能团相容,包括酮,酯,酰胺和卤代芳基。即使反应规模扩大到0.5克,也保持了高反应性。这是第一个铁催化的4-烯基异恶唑向吡咯的重排,与异恶唑底物第5位上烷氧基或氨基的存在无关。提出了铁介导的级联反应的合理机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号