当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-03-09 , DOI: 10.1039/c9bm01474h Jefferson O Abaricia 1 , Arth H Shah , Ryan M Musselman , Rene Olivares-Navarrete
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-03-09 , DOI: 10.1039/c9bm01474h Jefferson O Abaricia 1 , Arth H Shah , Ryan M Musselman , Rene Olivares-Navarrete
Affiliation
|
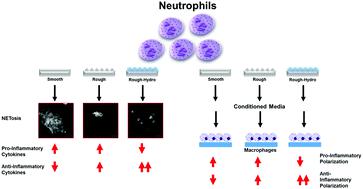
Biomaterial implantation triggers an immune response initially predominated by neutrophils, which activate an inflammatory cascade by producing cytokines, enzymes, immune cell recruitment chemokines, and DNA fiber networks called neutrophil extracellular traps (NETs). While the role of neutrophils has been studied extensively in infection, little is known of their role in the response to biomaterials, in this case titanium (Ti) implants. Furthermore, while implant surface modifications have been shown to attenuate pro-inflammatory polarization in other immune cells, their effects on neutrophil behavior is unknown. The aim of this study was to characterize the neutrophil response to Ti surface topography and hydrophilicity and understand how the products of biomaterial-induced neutrophil activation alters macrophage polarization. Murine neutrophils were isolated by density gradient centrifugation and plated on smooth, rough, and rough hydrophilic (rough-hydro) Ti surfaces. Neutrophils on rough-hydro Ti decreased pro-inflammatory cytokine and enzyme production as well as decreased NET formation compared to neutrophils on smooth and rough Ti. Conditioned media (CM) from neutrophils on smooth Ti enhanced pro-inflammatory macrophage polarization compared to CM from neutrophils on rough or rough-hydro Ti; pretreatment of neutrophils with a pharmacological NETosis inhibitor impaired this macrophage stimulation. Finally, co-culture of neutrophils and macrophages on Ti surfaces induced pro-inflammatory macrophage polarization compared to macrophages alone on surfaces, but this effect was ablated when neutrophils were pretreated with the NETosis inhibitor. These findings demonstrate that neutrophils are sensitive to changes in biomaterial surface properties and exhibit differential activation in response to Ti surface cues. Additionally, inhibition of NETosis enhanced anti-inflammatory macrophage polarization, suggesting NETosis as a possible therapeutic target for enhancing implant integration.
中文翻译:

亲水性钛表面可减少中性粒细胞炎症反应和 NETosis
生物材料植入会触发最初由中性粒细胞主导的免疫反应,后者通过产生细胞因子、酶、免疫细胞募集趋化因子和称为中性粒细胞胞外陷阱 (NET) 的 DNA 纤维网络激活炎症级联反应。虽然中性粒细胞在感染中的作用已得到广泛研究,但对它们在对生物材料(在这种情况下是钛 (Ti) 植入物)的反应中的作用知之甚少。此外,虽然植入物表面修饰已被证明可以减弱其他免疫细胞的促炎极化,但它们对中性粒细胞行为的影响尚不清楚。本研究的目的是表征中性粒细胞对 Ti 表面形貌和亲水性的反应,并了解生物材料诱导的中性粒细胞活化的产物如何改变巨噬细胞极化。鼠中性粒细胞通过密度梯度离心分离并镀在光滑、粗糙和粗糙的亲水(粗糙-水)钛表面。与光滑和粗糙 Ti 上的中性粒细胞相比,粗糙-hydro Ti 上的中性粒细胞减少了促炎细胞因子和酶的产生以及 NET 的形成。与来自粗糙或粗糙氢化钛上的嗜中性粒细胞的 CM 相比,来自平滑钛上的中性粒细胞的条件培养基 (CM) 增强了促炎性巨噬细胞极化;用药理学 NETosis 抑制剂预处理中性粒细胞会削弱这种巨噬细胞刺激。最后,与单独在表面上的巨噬细胞相比,Ti 表面上的嗜中性粒细胞和巨噬细胞的共培养诱导了促炎性巨噬细胞极化,但当嗜中性粒细胞用 NETosis 抑制剂进行预处理时,这种效应被消融。这些发现表明,中性粒细胞对生物材料表面特性的变化很敏感,并表现出对 Ti 表面线索的不同激活。此外,抑制 NETosis 增强了抗炎巨噬细胞极化,表明 NETosis 可能是增强植入物整合的治疗靶点。
更新日期:2020-04-24
中文翻译:

亲水性钛表面可减少中性粒细胞炎症反应和 NETosis
生物材料植入会触发最初由中性粒细胞主导的免疫反应,后者通过产生细胞因子、酶、免疫细胞募集趋化因子和称为中性粒细胞胞外陷阱 (NET) 的 DNA 纤维网络激活炎症级联反应。虽然中性粒细胞在感染中的作用已得到广泛研究,但对它们在对生物材料(在这种情况下是钛 (Ti) 植入物)的反应中的作用知之甚少。此外,虽然植入物表面修饰已被证明可以减弱其他免疫细胞的促炎极化,但它们对中性粒细胞行为的影响尚不清楚。本研究的目的是表征中性粒细胞对 Ti 表面形貌和亲水性的反应,并了解生物材料诱导的中性粒细胞活化的产物如何改变巨噬细胞极化。鼠中性粒细胞通过密度梯度离心分离并镀在光滑、粗糙和粗糙的亲水(粗糙-水)钛表面。与光滑和粗糙 Ti 上的中性粒细胞相比,粗糙-hydro Ti 上的中性粒细胞减少了促炎细胞因子和酶的产生以及 NET 的形成。与来自粗糙或粗糙氢化钛上的嗜中性粒细胞的 CM 相比,来自平滑钛上的中性粒细胞的条件培养基 (CM) 增强了促炎性巨噬细胞极化;用药理学 NETosis 抑制剂预处理中性粒细胞会削弱这种巨噬细胞刺激。最后,与单独在表面上的巨噬细胞相比,Ti 表面上的嗜中性粒细胞和巨噬细胞的共培养诱导了促炎性巨噬细胞极化,但当嗜中性粒细胞用 NETosis 抑制剂进行预处理时,这种效应被消融。这些发现表明,中性粒细胞对生物材料表面特性的变化很敏感,并表现出对 Ti 表面线索的不同激活。此外,抑制 NETosis 增强了抗炎巨噬细胞极化,表明 NETosis 可能是增强植入物整合的治疗靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号