当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: an experimental and theoretical study
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020-03-09 , DOI: 10.1039/d0na00137f Liuting Zhang 1 , Ze Sun 1 , Zhendong Yao 2 , Lei Yang 1 , Nianhua Yan 1 , Xiong Lu 1 , Beibei Xiao 1 , Xinqiao Zhu 3 , Lixin Chen 2
Nanoscale Advances ( IF 4.6 ) Pub Date : 2020-03-09 , DOI: 10.1039/d0na00137f Liuting Zhang 1 , Ze Sun 1 , Zhendong Yao 2 , Lei Yang 1 , Nianhua Yan 1 , Xiong Lu 1 , Beibei Xiao 1 , Xinqiao Zhu 3 , Lixin Chen 2
Affiliation
|
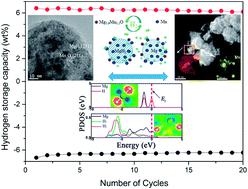
Recently, transition metal oxides have been evidenced to be superior catalysts for improving the hydrogen desorption/absorption performance of MgH2. In this paper, Mn3O4 nanoparticles with a uniform size of around 10 nm were synthesized by a facile chemical method and then introduced to modify the hydrogen storage properties of MgH2. With the addition of 10 wt% Mn3O4 nanoparticles, the MgH2–Mn3O4 composite started to release hydrogen at 200 °C and approximately 6.8 wt% H2 could be released within 8 min at 300 °C. For absorption, the completely dehydrogenated sample took up 5.0 wt% H2 within 10 min under 3 MPa hydrogen even at 100 °C. Compared with pristine MgH2, the activation energy value of absorption for the MgH2 + 10 wt% Mn3O4 composite decreased from 72.5 ± 2.7 to 34.4 ± 0.9 kJ mol−1. The catalytic mechanism of Mn3O4 was also explored and discussed with solid evidence from X-ray diffraction (XRD), Transmission Electron Microscope (TEM) and Energy Dispersive X-ray Spectroscopy (EDS) studies. Density functional theory calculations revealed that the Mg–H bonds were elongated and weakened with the doping of Mn3O4. In addition, a cycling test showed that the hydrogen storage capacity and reaction kinetics of MgH2–Mn3O4 could be favourably preserved in 20 cycles, indicative of promising applications as a solid-state hydrogen storage material in a future hydrogen society.
中文翻译:

Mn3O4纳米粒子对MgH2储氢性能的优异催化作用:实验和理论研究
最近,过渡金属氧化物已被证明是改善MgH 2的氢解吸/吸氢性能的优良催化剂。在本文中,通过简便的化学方法合成了约10 nm 的均匀尺寸的Mn 3 O 4纳米粒子,然后将其引入以改变MgH 2的储氢性能。添加 10 wt% Mn 3 O 4纳米颗粒后,MgH 2 -Mn 3 O 4复合材料在 200 °C 开始释放氢,大约 6.8 wt% H 2可在 300 °C 下 8 分钟内释放。对于吸收,即使在 100 °C 下,完全脱氢的样品在 3 MPa 氢气下 10 分钟内吸收了 5.0 wt% H 2 。与原始MgH 2相比,MgH 2 + 10 wt% Mn 3 O 4复合材料的吸收活化能值从72.5 ± 2.7 降低到34.4 ± 0.9 kJ mol -1。Mn 3 O 4的催化机理还利用来自 X 射线衍射 (XRD)、透射电子显微镜 (TEM) 和能量色散 X 射线光谱 (EDS) 研究的可靠证据进行了探索和讨论。密度泛函理论计算表明,随着Mn 3 O 4的掺杂,Mg-H键被拉长和减弱。此外,循环测试表明,MgH 2 -Mn 3 O 4的储氢容量和反应动力学可以在20个循环内保持良好,表明在未来氢社会中作为固态储氢材料的应用前景广阔。
更新日期:2020-04-24
中文翻译:

Mn3O4纳米粒子对MgH2储氢性能的优异催化作用:实验和理论研究
最近,过渡金属氧化物已被证明是改善MgH 2的氢解吸/吸氢性能的优良催化剂。在本文中,通过简便的化学方法合成了约10 nm 的均匀尺寸的Mn 3 O 4纳米粒子,然后将其引入以改变MgH 2的储氢性能。添加 10 wt% Mn 3 O 4纳米颗粒后,MgH 2 -Mn 3 O 4复合材料在 200 °C 开始释放氢,大约 6.8 wt% H 2可在 300 °C 下 8 分钟内释放。对于吸收,即使在 100 °C 下,完全脱氢的样品在 3 MPa 氢气下 10 分钟内吸收了 5.0 wt% H 2 。与原始MgH 2相比,MgH 2 + 10 wt% Mn 3 O 4复合材料的吸收活化能值从72.5 ± 2.7 降低到34.4 ± 0.9 kJ mol -1。Mn 3 O 4的催化机理还利用来自 X 射线衍射 (XRD)、透射电子显微镜 (TEM) 和能量色散 X 射线光谱 (EDS) 研究的可靠证据进行了探索和讨论。密度泛函理论计算表明,随着Mn 3 O 4的掺杂,Mg-H键被拉长和减弱。此外,循环测试表明,MgH 2 -Mn 3 O 4的储氢容量和反应动力学可以在20个循环内保持良好,表明在未来氢社会中作为固态储氢材料的应用前景广阔。











































 京公网安备 11010802027423号
京公网安备 11010802027423号