当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselectivity of hyoscyamine 6β-hydroxylase-catalysed hydroxylation as revealed by high-resolution structural information and QM/MM calculations
Dalton Transactions ( IF 3.5 ) Pub Date : 2020/03/09 , DOI: 10.1039/d0dt00302f Anna Kluza 1 , Zuzanna Wojdyla 1 , Beata Mrugala 1 , Katarzyna Kurpiewska 2 , Przemyslaw J Porebski 3 , Ewa Niedzialkowska 3 , Wladek Minor 4 , Manfred S Weiss 5 , Tomasz Borowski 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2020/03/09 , DOI: 10.1039/d0dt00302f Anna Kluza 1 , Zuzanna Wojdyla 1 , Beata Mrugala 1 , Katarzyna Kurpiewska 2 , Przemyslaw J Porebski 3 , Ewa Niedzialkowska 3 , Wladek Minor 4 , Manfred S Weiss 5 , Tomasz Borowski 1
Affiliation
|
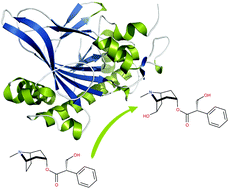
Hyoscyamine 6β-hydroxylase (H6H) is a bifunctional non-heme 2-oxoglutarate/Fe2+-dependent dioxygenase that catalyzes the two final steps in the biosynthesis of scopolamine. Based on high resolution crystal structures of H6H from Datura metel, detailed information on substrate binding was obtained that provided insights into the onset of the enzymatic process. In particular, the role of two prominent residues was revealed – Glu-116 that interacts with the tertiary amine located on the hyoscyamine tropane moiety and Tyr-326 that forms CH–π hydrogen bonds with the hyoscyamine phenyl ring. The structures were used as the basis for QM/MM calculations that provided an explanation for the regioselectivity of the hydroxylation reaction on the hyoscyamine tropane moiety (C6 vs. C7) and quantified contributions of active site residues to respective barrier heights.
中文翻译:

高分辨率结构信息和 QM/MM 计算揭示了天仙子胺 6β-羟化酶催化羟基化的区域选择性
Hyoscyamine 6β-hydroxylase (H6H) 是一种双功能非血红素 2-oxoglutarate/Fe 2+依赖性双加氧酶,可催化东莨菪碱生物合成的最后两个步骤。基于曼陀罗H6H 的高分辨率晶体结构,获得了底物结合的详细信息,从而深入了解酶促过程的开始。特别是揭示了两个突出残基的作用——Glu-116 与位于天仙子胺托烷部分的叔胺相互作用,Tyr-326 与天仙子胺苯环形成 CH-π 氢键。这些结构被用作 QM/MM 计算的基础,它提供了羟基化反应对堇青素托烷部分的区域选择性的解释(C6对. C7) 并量化了活性位点残基对各自势垒高度的贡献。
更新日期:2020-04-08
中文翻译:

高分辨率结构信息和 QM/MM 计算揭示了天仙子胺 6β-羟化酶催化羟基化的区域选择性
Hyoscyamine 6β-hydroxylase (H6H) 是一种双功能非血红素 2-oxoglutarate/Fe 2+依赖性双加氧酶,可催化东莨菪碱生物合成的最后两个步骤。基于曼陀罗H6H 的高分辨率晶体结构,获得了底物结合的详细信息,从而深入了解酶促过程的开始。特别是揭示了两个突出残基的作用——Glu-116 与位于天仙子胺托烷部分的叔胺相互作用,Tyr-326 与天仙子胺苯环形成 CH-π 氢键。这些结构被用作 QM/MM 计算的基础,它提供了羟基化反应对堇青素托烷部分的区域选择性的解释(C6对. C7) 并量化了活性位点残基对各自势垒高度的贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号